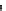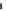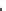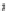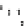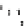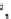Biology Reference
In-Depth Information
D1
D2
D3
D4
D5
a
b
MMP
26.9Å
c
d
90
o
C-telopeptide
Collagenase
[C]
O
e
[N]
I
overlap
gap
D-period (67 nm)
Fig. 5.6 Structure of a collagen fibril. The structural organization of a collagen fibril is shown.
(a) Schematic representation of a fibril showing the staggered arrangement of individual
collagen molecules. One molecule is highlighted in a
rainbow colour
scheme from
blue
at the
N-terminus to
red
at the C-terminus. (b) The above stagger gives collagen fibrils/fibres their
characteristic D-periodicity banding pattern. (c) Orgel et al. (
2006
) solved the structure of rat tail
type I collagen fibrils by fibre diffraction. This gave direct insight in the fibril organization and
its accessibility for collagenase (Perumal et al.
2008
). The structure is of a D-repeat in an
orientation as if one is looking down onto the fibril. The colour-coding is the rainbow scheme as
before with each collagen molecule
blue
at the N-terminus and
red
at the C-terminus. (d)The
grey
panels represent each cut-through section through the fibril showing the orientation of the
individual collagen molecules relative to each other using the same colour scheme. The MMP-
cleavage site is indicated by “X.” This site is mostly covered by the C-terminal telopeptides of
the adjacent collagen molecule, thereby severely limiting the access for collagenase. (e)The
view of five collagen molecules within the D5 period. The
top
is the fibrilar surface. Note that the
N-terminus (
dark blue
) of collagen molecule is below the surface of the fibril and gradually
reaches to the surface in the gap region (
orange
) and terminates with the C-terminal telopeptide
(
red
). The collagenase-cleavage site (shown is while in the
orange molecule
) is covered by the
C-terminal telopeptide by another collagen molecule about it. The figure was made with Pymol
(Coordinates courtesy of J.P.R.O. Orgel.)
molecules are arranged to form a supertwisted, discontinuous, right-handed micro-
fibril that interdigitates with neighbouring microfibrils (see Fig.
5.6c-e
). This
structure indicates that the collagenase cleavage site in the collagen molecule is
largely blocked by the C-telopeptide of the neighbouring collagen molecules
(Perumal et al.
2008
). This suggests that proteinases that have telopeptidase activity