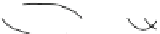Biology Reference
In-Depth Information
However, chimeras consisting of the N-terminal part of the MMP-3 catalytic
domain and the rest of MMP-1 (LC3) exhibited about fourfold higher unwinding
activity and about three- to fivefold higher cutter activity, but the active full-length
enzyme was a very poor collagenase, suggesting that the unwound
-chains are
probably not aligned well within the active site of the chimeric full-length enzymes.
Thus, we postulate that for a collagenase to cleave collagen, multiple steps are
required during the unwinding process before it finally cleaves the peptide bonds.
These steps may be important in collagen specificity, which is dictated by both the
collagen sequence and enzyme's unwinding properties. Recent studies of Han et al.
(
2010
) have shown that
a
a
1(I)
3
homotrimers are far more resistant to collagenases
than
2(I) heterotrimers and this is due to less efficient unwinding of
homotrimers by MMP-1. The multiple step model of collagen unwinding is illu-
strated in Fig.
5.5
together with the model proposed by Stultz and colleagues
(Nerenberg et al.
2008
).
a
1(I)2
a
a
b
Zn
2+
Hpx
Zn
2+
Hpx
Zn
2+
Hpx
(i)
Zn
2+
Hpx
Zn
2+
Hpx
(ii)
Zn
2+
Zn
2+
Hpx
Hpx
(iii)
Zn
2+
Zn
2+
Hpx
Hpx
Fig. 5.5 Models of collagen cleavage. (a) A collagenase-induced unwinding model. This model
involves multiple steps: (i) Collagenase (
light grey
represents the catalytic domain and
dark grey
the Hpx domain) binds to native triple helical collagen with the cleft formed between the Cat and
Hpx domains; (ii) the collagen is locally unwound though the contact with collagenase; and (iii)
the fitting of the unwound strand to the active site. This is followed by cleavage of the three strands
of collagen. (b) The Stultz model. In this model, local unfolding of collagen around the cleavage
site allows collagenase to bind and cleave