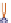Agriculture Reference
In-Depth Information
Mode of action of disinfectants
Oxidising:
halogens, peroxides,
alkylating: aldehydes
coagulating: alcohols
Radical forming actives:
halogens, isothiazolones, peroxides?
Alkylating: aldehydes
(Per-)acids
Perforation:
QAC, biguanides,
amines, glucoprotamines
Reorganisation:
polymeric biguanides, alcohols
PMF
DNA
Plasma
(proteins)
Microbial cell
Protein
Cell membrane
Cell wall
Cell membrane
(double layer of lipids)
PMF = proton-motive forces
Cell wall
Figure 5.3
Action of biocides on bacteria (Reproduced with permission from Ecolab. © Ecolab).
function is to kill bacteria and other micro-organisms
that are left on the surface after cleaning. They can kill
microbes by several different methods, depending on
which components are used in the disinfectant. Some
affect the integrity of the cell wall, while others interfere
with critical metabolic reactions inside the cell (Fig. 5.3).
Scientific understanding of the mechanisms of action is
well described and gaining focus driven by the need to
understand the phenomenon of resistance (Maillard,
2002; McDonnell and Russell, 1999).
Some disinfectants are
oxidising
and will tend to react
with most organic material, whether meat residues or
bacteria. These oxidising disinfectants include
chlorine
,
iodophors
and
peracids
. These agents are usually rapid
acting and broad spectrum in terms of the organisms
they can kill inclusive of their spores, but they typically
lack a residual effect. They may not be stable in hot water
and may be corrosive on a range of metals and other sur-
faces, but they are usually low foaming.
It is sometimes wrongly assumed that a chlorine foam
can act
fully
as a cleaner and a disinfectant and that sub-
sequent disinfection is not needed. This is partly a false
assumption, based on the perception of chlorine as a
disinfectant. Depending on the pH, there is an equilib-
rium in chlorine solutions between the hypochlorous
acid (HOCl) molecule and the hypochlorite ion (OCl
−
).
The main active biocide in chlorine release agents is
the hypochlorous acid molecule HOCl; it is uncharged
and for this reason is thought to penetrate the cell walls
of microbes more easily. In chlorine foam cleaners,
the application solution is usually around pH 10-11.
The chlorine is therefore mostly present as the hypochlo-
rite ion OCl
−
, which acts principally as a detergent and
oxidising agent, helping with the removal of proteins
and grease and the bleaching of some coloured sub-
stances. This pH effect and lack of free HOCl makes the
disinfectant properties of alkaline chlorine solutions
much weaker (up to a hundred times) than a straight
hypochlorite disinfectant solution without alkalis. The
better cleaning performance of the chlorine foam physi-
cally removes much of the bacterial load along with the
dirt, but in areas where a very low surface bacterial
count is desired, a separate disinfection stage is needed.
This should normally not be a hypochlorite solution
because of the risk of corrosion (even on stainless steel)
from the breakdown products of the hypochlorite and
product taint from poorly rinsed surfaces. The lack of
heat and light stability of the chlorine can mean that
no residual bactericidal effect is maintained after a
relatively short time.
Non-oxidising
disinfectants are typically based on
quaternary ammonium compounds or 'quats' - a class of
cationic surfactants and amphoterics - another class of
surfactants with twin positive and negative charges, alco-
hols, biguanides or aldehydes. The non-oxidising agents
are usually heat stable and less corrosive and have a
residual biocidal or biostatic effect if left on surfaces. The
surfactant-based disinfectants are often foaming, which