Biomedical Engineering Reference
In-Depth Information
3 Na
3 Na
3 Na
E
1
-ATP
E
1
-ATP
E
1
P-ADP
+
2K
+
Na
ADP
+
3Na
2 K
2 Na
E
1
-ATP
+
2K
ATP
+
2Na
H
2
O
P
1
2 K
2 K
2 K
E
2
.P
1
E
2
E
2
P
The reaction cycle of the Na
+
,K
+
-ATPase [
23
,
40
]
Fig. 4.13
[
22
,
32
]. A FXYD family member protein often associates itself with the
αβ
-complex
as a third subunit, and regulates the pumping activity in a tissue- and isoform-specific
way [
20
,
51
].
A recent study of the crystal structure on the sodium-potassium pump [
36
] has
described clearly how the membrane component is relevant to the activity of the
pump. In light of the sensitivity of the Na
+
,K
+
-pump activity to the membrane
potential, it is notable that arginines make the area around the C-terminus in the mem-
brane edge region highly electropositive. In various types of voltage-dependent ion
channels, arginine clusters act as voltage sensors that move in response to membrane
depolarization, and in the Na
+
,K
+
-ATPase the arginine cluster associated with the
C-terminus could function similarly as a control point for a voltage-sensitive switch
that alters the relations of the C-terminus in its binding pocket during depolariza-
tion/repolarization, with consequences for the Na
+
affinity. The hypothesis involving
a direct structural and functional relation between the C-terminus and the third Na
+
site is in accordance with the high voltage-sensitivity of the binding and release of
one of the three Na
+
ions [
4
,
19
].
References
1. Andersen, O.S., Sawyer, D.B., and Koeppe II, R.E.: Bio membrane structure and Function.
edited by K. R. K. Easwaran and B. Gaber (Schenectady, New York: Adenine), 227-244
(1992)








































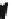












Search WWH ::

Custom Search