Biomedical Engineering Reference
In-Depth Information
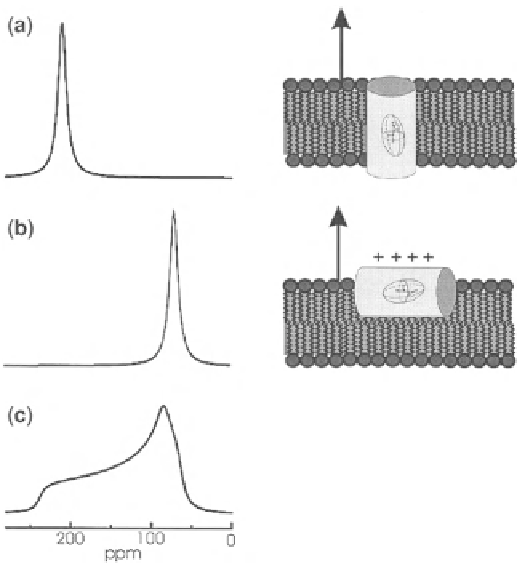
Fig. 4.10
Calculated proton-decoupled 15N solid-state NMR spectra of helical peptides
15N-labeled at a single site and reconstituted into oriented membranes (illustrated to the
right
of the spectra). The membrane normal is aligned parallel to the magnetic field direction (
arrows
).
a
Trans-membrane orientation of the helix axis.
b
Orientation of the peptide approximately per-
pendicular to the magnetic field direction.
c
Powder pattern line shape where a random distribution
of molecular orientations has been assumed. This figure has been adapted from [
13
] with due
permission from the publisher
fields. This group also suggested, through convincing analysis, that the lipid polar
groups act as 'molecular electrometers' that respond to both molecules partitioning
into the lipid bilayers, and any process that modifies the electrical properties of the
membrane surface. Earlier, the dipole moment of the phosphocholine group was cal-
culated, which, in its 200 declination with the membrane surface, was found to induce
a very high 90 mV dipole potential in the membrane environment [
46
,
47
]. Depending
on the direction of the dipole moment, the potential can enhance or reduce the existing
electric potential, and is sufficiently large to trigger conformational and/or functional
changes in membrane proteins, or to facilitate peptide or protein insertion into the
membrane's hydrophobic core. The membrane absorption of peptides bearing elec-
trostatic charges therefore substantially influences the functions of these molecular
electrometers, with the lipids having reverse effects on the peptide aggregation or
any general function. From the discussion presented above, it is clear that all kinds of
lipids may cause localized charge effects, and these effects become complex with the
Search WWH ::

Custom Search