Biomedical Engineering Reference
In-Depth Information
Fig. 4.3
Barrel-stave model
for alamethicin channel for-
mation inside lipid bilayers
[
8
,
15
,
26
].
Cylindrical rods
are schematic diagrams for
alamethicin monomers in 3D
view
Fig. 4.4
The transition
between different conduc-
tion pores of alamethicin
channels
c
i
c
i+1
c
i+2
between different energy states in a barrel-stave pore occur (Figs.
4.3
and
4.4
) has
recently been offered [
6
]. A more detailed discussion of the stability, energetics, and
4.2 Lipid-Lined Ion Channels in Membranes
In lipid-lined channels, it is generally assumed that channel-forming peptides interact
with the lipid membrane, which creates the alignment of lipids along the channels.
Ions flow through the opening between cellular exterior and interior regions, and
possibly through the lipid regions, avoiding the peptides involved in creating chan-
nels. The lipid alignment causes an opening which may look like a long cylindrical
lipid-aligned channel, where the membrane thickness may not change dramatically.
The other possibility is that the membrane thickness slowly vanishes at the channel
opening, which can equivalently be considered as a broken membrane condition. In
many investigations this broken membrane structure has been predicted as a model
for the lipid lined channels. Figure
4.5
shows a schematic diagram of this situa-
tion. This kinds of structures are found to be induced by both AMPs, e.g., magainin
[
31
,
34
], melittin [
57
], colicin [
50
], etc., or by other non-antimicrobial agents, such
as the recently discovered pores by chemotherapy drugs thiocolchicocide and taxol
molecules [
7
]. The magainin-, melittin- and colicin-induced toroidal pores can also
be considered to be protein-lined channels, because here the peptides are always
associated with the lipid head groups, with the result that the lipid monolayers bend

























































































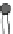








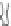


































































































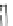
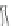



































































































Search WWH ::

Custom Search