Biomedical Engineering Reference
In-Depth Information
3.5
gA-PS
gA-PC
3.0
2.5
2.0
1.5
1.0
0.5
0.0
0
-20
E
vdW
E
ES
-40
-60
4000
100
3000
-80
0
2000
-100
1000
-100
-200
0
5 1015202530
51015202530
-120
d
drug-lipid
(Å)
d
drug-lipid
(Å)
1.4
ALA-PC
ALA-PS
1.2
1.0
0.8
0.6
0.4
0.2
0.0
0
-6
1.5
160
1.0
-12
80
0.5
0
0.0
-80
-0.5
-160
-1.0
-18
-240
-1.5
6
8
1 0
6
8
10
d
drug-lipid
(Å)
d
drug-lipid
(Å)
-24
6
8
10 12 14 16 18 20 22 24 26 28 30
6
8
10 12 14 16 18 20 22 24 26 28 30
d
drug-lipid
(Å)
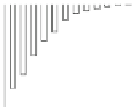
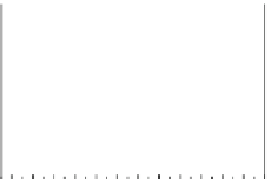
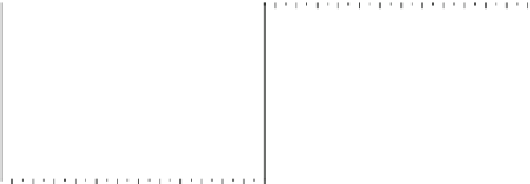
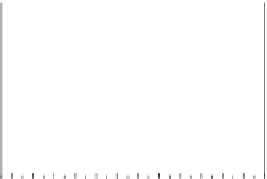
Fig. 5.27
In all the histograms (
upper panel
)oftimeversus
d
drug
−
lipid
, the duration of the drug/lipid
complex staying together (height) within a distance (width) in 6 ns MD simulations is presented.
Lower panels
show the histograms of non-bonded van der Waals (vdW) energy (
E
vdW
) and elec-
trostatic (ES) interaction energy (
E
ES
). To avoid color conflict,
E
vdW
and
E
ES
are shown to occupy
half-half widths though each half represents the whole width of the corresponding histogram
Other studies using
β
-helical gramicidin A channels [
7
] and
α
-helical peptides like
acetyl-GWW(LA)
n
LWWA-amide (WALP) [
47
,
48
], incorporated in lipid bilayers
with different thicknesses, provide experimental evidence for the response between
bilayer and protein structural alterations and the hydrophobic mismatch. An increase
in the values of
G
I
,
II
causes destabilization of the corresponding channels. There-
fore, the stability or the average lifetime of a channel can decrease by decreasing










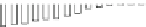



































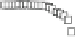





























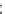

































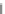














































































































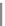






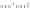
















































































































































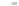



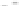





















































































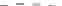




































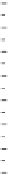



























































Search WWH ::

Custom Search