Biomedical Engineering Reference
In-Depth Information
Fig. 5.8
Barrel-stave model for alamethicin channel formation inside lipid bilayers [
14
,
19
,
36
].
Cylindrical rods are schematic diagrams for alamethicin monomers in 3D view
interaction sites, but the channel may still experience slight destabilization due to
any possible fluctuation in
G
prot
.
5.2.2 Screened Coulomb Interactions and Lennard-Jones
Potentials Between Peptides on Ion Channels and Lipids
on the Membrane: A Study Using an Alamethicin
Channel as a Tool
In the previous section, we have learned that the bilayer-spanning gramicidin A
channels are formed by the reversible, trans-bilayer association of
3
-helical
gramicidin A monomers [
68
]. Alamethicin channels form 'barrel-stave' type pores
[
14
,
19
,
36
] where the alamethicin monomers align across the cylindrical surface
of the channel with many possible conductance states, depending on the number of
alamethicin monomers involved in the formation of a cylindrical channel (see the
proposed alamethicin channel model in Figs.
5.8
,
5.9
and
5.10
).
6
.
β
Extrapolation of Gramicidin A Channel Energetics to the Alamethicin
Channel
The form of
V
sc
(Eq.
5.14
) in the case of alamethicin channels is still the same, but
instead of considering only two screened Coulomb interactions between the channel
and the bilayer at the two longitudinal ends of the channel as is considered in the
gramicidin A channel's interaction sites with the bilayer, each alamethicin monomer





































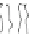

















































































































































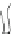
















































Search WWH ::

Custom Search