Biomedical Engineering Reference
In-Depth Information
liquid droplet
gas−liquid
surface tension
gas
virtual line
gas−solid
surface tension
solid
liquid−solid
surface tension
contact angle
Fig. 13.2
Forces acting on a liquid droplet in a gas wetting a solid. Small droplets (size of
O
[1 mm]) are spherical whereas larger ones are ellipsoidal under the effect of gravity.
surface phenomena.
6
The surface molecules then bear a net attraction toward the
liquid body. The centrally directed forces minimize both the free energy
7
and the
surface area. The surface tension expresses cohesion internal forces that hinder the
liquid molecules from occupying the available space.
Two fluids in contact, such as a liquid and its vapor, are separated by an interface.
The interface properties differ from those of 2 uniform phases. The interface is
modeled by a zero-thickness membrane undergoing an uniform tension. Let us
define 2 surface regions separated by a virtual line on the surface (Fig.
13.2
). In any
point of this line, the length unit
ds
is subjected to a force from one of the 2 regions,
normal to the line and tangent to the surface at the selected point, of magnitude:
df
=
T
s
ds
,
(13.1)
T
−
2
;N/m).
8
The surface tension decays when
the temperature rises and becomes zero at the critical temperature. A surface energy
(
W
) corresponds to the surface force on the surface area (
A
):
W
where
T
s
is the surface tension (
M
.
T
s
A
.
At the beginning of the nineteenth century (1805-1806), T. Young and P.S. de
Laplace found the equation that describes the relationship between the curvature
=
6
The cohesion forces are more important in liquids than in gases, with smaller molecule
concentration. The higher the cohesion forces of a liquid, the stronger the surface tension, and
the lower the liquid wetting.
7
The total free energy of a system composed of 2 uniform fluids of densities ρ
1
and ρ
2
, of volumes
V
1
and
V
2
, and of specific free energy
e
1
and
e
2
, separated by an interface of area
A
is given by:
e
tot
=
ρ
1
V
1
e
1
+
ρ
2
V
2
e
2
+
T
s
A
,
where
T
s
A
represents the surface energy,
T
s
being the surface tension that can hence be interpreted
as free energy per unit area of the interface. The interface stretching work done by the surface
tension in small reversible isothermal changes in the fluid system is equal to the gain in total free
energy.
8
The surface tension between air and water is equal to 73.10
−
3
N/m at 20
◦
C (293 K) and to
68.10
−
3
N/m at 50
◦
C (323 K).






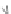

Search WWH ::

Custom Search