Environmental Engineering Reference
In-Depth Information
of automobile catalytic converters, leading to increased tailpipe emissions of both nitrogen
oxides (NOx) and CO
2
.
Governments worldwide have responded by enacting laws to restrict the quantity of
sulfur allowed in fossil fuels, primarily those intended for transportation. In the past 10 years,
allowable levels of sulfur in transportation fuels have diminished from 2,000-5,000 parts per
million (ppm) to less than 500 ppm; recent regulations proposed by the Directive of the
European Parliament [4] and the EPA [5] will lower these levels to below 350 ppm. By 2010,
even lower restrictions (less than 10-15 ppm in practice) are expected [1].
1.1. Hydrodesulfurization
The primary conventional technology used to remove sulfur from crude oil is
hydrodesulfurization, or HDS. By subjecting crude oil to elevated temperatures and hydrogen
partial pressure in the presence of a CoMo/Al
2
O
3
or NiMo/Al
2
O
3
catalyst, reactive sulfur
components such as mercaptans, sulfides, and disulfides are converted to H
2
S and
hydrocarbons. Lower boiling point fractions of crude oil contain primarily these aliphatic
organosulfur compounds and are therefore desulfurized with great success by HDS. In higher
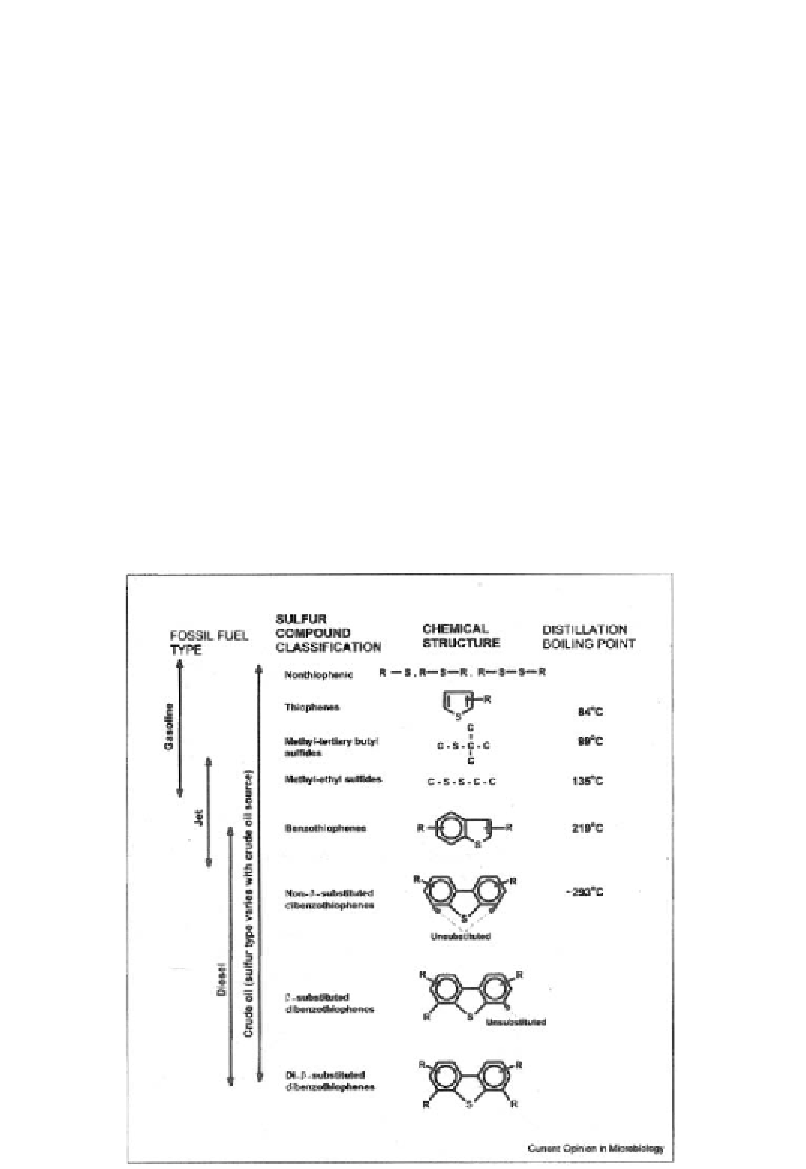
boiling point fractions, however, the organosulfur compounds primarily contain thiophenic
rings, including thiophenes, benzothiophenes, and their alkylated derivatives. Unfortunately,
HDS is much less efficient in the desulfurization of these compounds (Figure 20), with the
result that so-called deep desulfurization technologies are being actively explored [7].
Figure 20. Organosulfur compounds present in fossil fuels [7].