Biology Reference
In-Depth Information
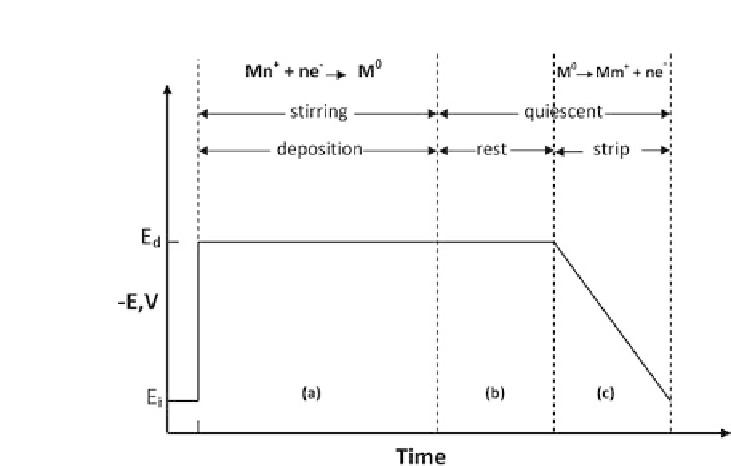
Figure 8.5.
Potential-time waveform used in ASV. (a) Deposition of metal
ions. (b) Rest period to allow solution to become quiscent. (c) Potential is
driven positive of the oxidation potential of the metal film.
film (MFE). The latter case is produced by reducing a layer of
mercury (thickness
∼
1-1000 nm) onto a solid electrode. This can
be done conveniently by adding mercury ions (10
−
5
M-10
−
4
M)
to the analyte solution, so that the MFE forms during the analyte
preconcentration. Where the analyte has an oxidation potential
more positive than mercury (e.g., Ag or Au) a solid electrode must
be used. Screen-printed carbon electrodes have been successfully
applied to the ASV detection of metal-nanoparticle labels ([31],
[36]), although obviously a screen-printed electrode surface is less
reproducible than that of mercury. Where such electrodes are used
(and for that matter MFEs) the stripping step will remove virtually
allofthedepositedmaterial,resultinginarelativelysharppeak.This
characteristic, combined with the fact that
E
0
is unique for each
metal, enables multianalyte detection from a single voltammogram.
Such voltammograms can thus be applied to the simultaneous
detectionofmorethanoneDNAsequencebyusingadifferentmetal
labelfor each sequence [44].