Biology Reference
In-Depth Information
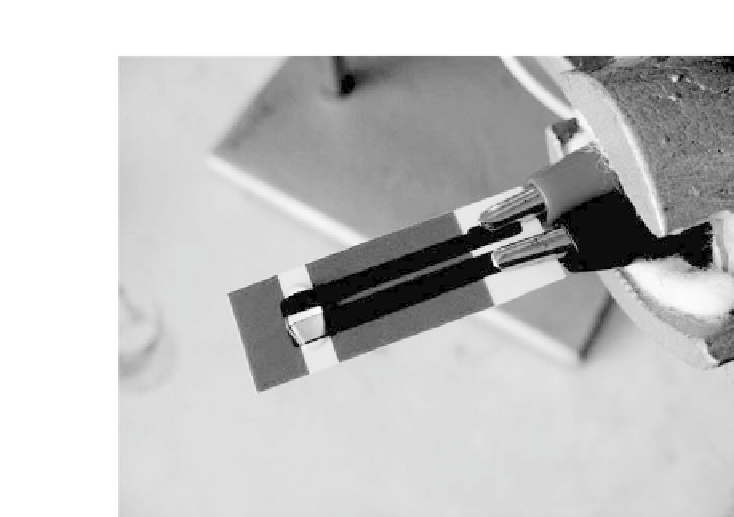
Figure 8.4.
Screen-printedtwoelectrodecellusedforssDNAimmobiliza-
tion. The carbon track working electrode is held at 100 mV vs. the Ag/AgCl
track reference/counter electrode for 30 s in the presence of 20
μ
L ssDNA
solution. See also ColorInsert.
of microamperes or less. An example of such a cell is the screen-
printed electrode strip shown in Fig. 8.4, which was used by us
to immobilize target DNA. The advantage of such a system is (a)
disposability, and (b) only a small volume of electrolyte is needed
to complete the cell, and therefore only a small quantity of DNA is
required.
The redox current is related to the charge
Q
passed during the
reaction by
I
=
d
Q
/d
t
. That charge is connected to the quantity
of material reacting by Faraday's law,
Q
=
mnF
,where
m
is
the number of moles converted,
F
is Faraday's constant, and
n
is the stoichiometric number of electrons. Equating the material
consumption, the flux of electrons at the electrode must be equal
tothefluxofthereactingspecies.ThisfluxisdescribedbyFick's1st
law. Hence, the electrode current is related to the concentration of