Biology Reference
In-Depth Information
7.3.1
Direct DNA Electrochemistry
Native electrochemical properties of DNA were first described by
Palecek [25]. The oxidations of either guanine and adenine are
irreversible multistep processes (see Fig. 7.3) [26-28].
Unfortunately, the oxidation of nucleobases is not desirable
under normal circumstances as it often results in the formation
of reactive species that lead to DNA decomposition. For instance,
guanine can be electrochemically oxidized [29], but practical
application of guanine oxidation as detection method is limited
to the use of G-free capture strands. Nevertheless, despite high
oxidation potentials and irreversibility of the oxidation process
several interesting mismatch detection schemes based on direct
nuclobase electrochemistry are worth mentioning. For instance,
Napier
et al.
[30] demonstrated the detection of the hybridization
ofproductsofthepolymerasechainreactionusingelectrontransfer
fromguaninetoatransition-metalcomplex.Thehybridizationassay
involved recording of cyclic voltammograms of [Ru(bpy)
3
]
2
+
(bpy,
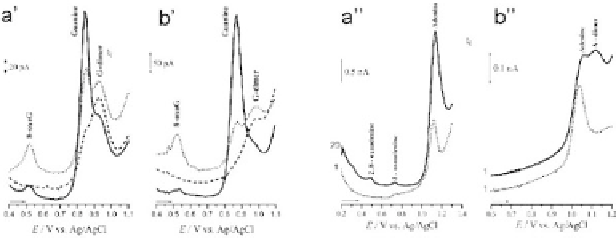
Figure 7.3.
Differential pulse voltammetry of guanine ( 5th scan) at
pH 4.5 in 0.2 M acetate buffer at a glassy carbon microelectrode. (a')
0.5 mM guanine; (b') 50
μ
M guanine (
...
1st scan;
−−−
2nd scan, after
transferring the microelectrode to supporting electrolyte) at a scan rate
of 5 mV/s. Differential pulse voltammetry of adenine ( ) at pH 4.5 in 0.2
M acetate buffer at a glassy carbon microelectrode. (a”) 1 mM adenine;
(b”) 10
μ
M adenine (
...
1
st
scan, after transferring the microelectrode
to supporting electrolyte) at a scan rate of 5 mV/s. Reprinted from
Bioelectrochemistry
,
55
,A.M.Oliveira-Brett,V.DiculescuandJ.A.P.Piedade,
Electrochemicaloxidationmechanismofguanineandadenineusingaglassy
carbon microelectrode, 61-62, 2002, with permission from Elsevier.