Biology Reference
In-Depth Information
conventional heterogeneous catalysis [12] and in general, this is a
process that occurs at the molecular or atomic level independent
of the catalyst dimensions [6, 14]. There is a considerable amount
of research articles and interesting reviews in what concerns to
the study of nanoparticle-catalyzed reactions, but the application
of these reactions in electrochemical analysis is not so well
documented.
Employing NPs in electroanalysis can induce more sensitive
and selective sensors as well as more cost-effective and portable
systems. Their application as catalysts in electroanalytical systems
can decrease overpotentials of many important redox species,
inducing discrimination between different electroactive analytes,
and also allowing the occurrence and reversibility of some redox
reactions, which are irreversible at common modified electrodes
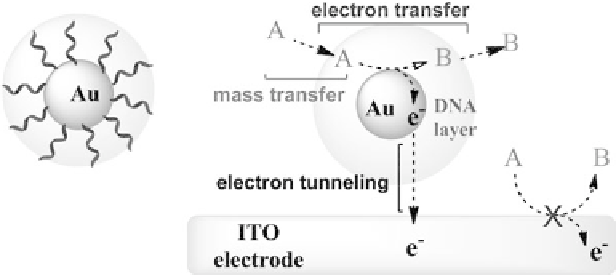
[15]. The catalytic effect can be explained through the enhancement
ofelectrontransferbetweentheelectrodesurfaceandthespeciesin
solution,byenhancementofmasstransportoralsobytheNPs'high
surfaceenergythatallowsthepreferredadsorptionofsomespecies
that by this way suffer achange in their overpotentials (Fig. 5.1).
The most exploited materials in catalysis are the metals from
platinum group, but with the introduction of nanotechnology some
other elements that in bulk state did not attract a lot of attention,
either due to their lack of reactivity toward some analytes or due to
their highcosts in production, are now emerging.
Figure 5.1.
Schematic illustration of the processes that affect the
electrocatalytic oxidation by Au-NP when functionalized with DNA strands
(adapted from Ref. 7 with permission). See also Color Insert.