Geoscience Reference
In-Depth Information
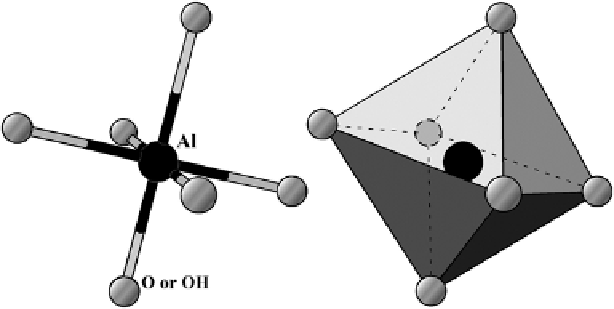
Fig. 5.5
The schematic demonstration of Al octahedron in minerals
higher than that between O-Ca-O. The olivine group with a ring arrangement of
silicon tetrahedrals has 10 B bridges in one structural unit. Small differences in its
easily weathered characteristic are caused by its various kinds of central ions.
Pyroxenes with 8 B bonds have single chains of tetrahedrons linked together with
octahedrons. Their weathering is less intensive when compared to minerals of the
olivine group. Amphiboles having two chains of tetrahedrons linked together in a
unit containing only 6 B bonds are more resistant to weathering than pyroxenes. If
the tetrahedrons are linked into a layer, groups of mica minerals are formed with 4
B bonds that are diffi cult to weather. On the other hand, their weathering proceeds
a little more readily if they exist in very thin sheets. Having bonds of only O-Si-O,
quartz has the general formula of SiO
2
. With octahedrons always missing and in the
total absence of any B bonds, each of its oxygen atoms is shared between two tetra-
hedrons linked together in all three dimensions. This confi guration is why minerals
of quartz group are not weathered (Fig.
5.6
).
When we return to our example of scaffolding at the start of this chapter, B bonds
could be modeled as thinner, even damaged tubes. It is obvious that thin previously
damaged tubes will be the fi rst to break when hit by stormy winds, and at the
moment they break, they cause a gradual collapse of the whole scaffolding. The
higher the number of those broken and unattached tubes, the earlier the collapse
occurs. If there were only one or two such tubes, the scaffolding would survive the
windstorm without visible damage.
Let us now return to our discussion of crystal lattice weathering. In real minerals
the shapely, neat regularity of the described models is disturbed by substitutions of
their central cations. For example, a three-valent aluminum cation could take the
place of a four-valent silicon cation within a tetrahedron. In spite of their very
similar sizes, the stability of such a tetrahedron is disrupted. In octahedrons the
central aluminum could be substituted by a two-valent iron or calcium cation
accompanied by a loss of stability in a manner similar to the result of substitution in
a tetrahedron (Fig.
5.5
).