Geoscience Reference
In-Depth Information
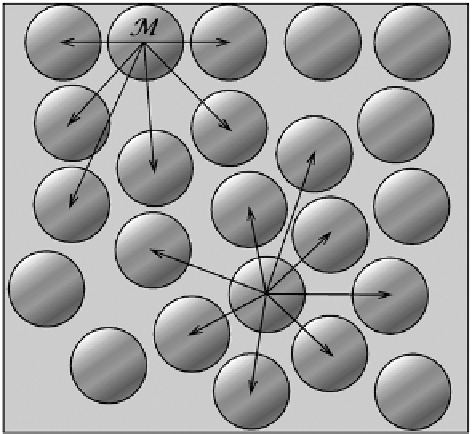
Simplifying molecular conditions in the vicinity of a horizontal, fl at plain of
water, we arbitrarily designate in Fig.
8.5
any one of the uppermost water molecules
along the plain with a script
M
. Such a molecule is attracted to molecules located
below and horizontally around it. Horizontally, the attraction from a molecule on its
right side is annihilated by that from its left side, and the same happens to all mol-
ecules on the surface. Hence, horizontally placed molecules have no effect upon our
designated molecule
M
at the water level. Because there are no water molecules
above our molecule
M
, the top half of it is not upwardly attracted. Cohesion is there-
fore restricted to the bottom part of our molecule
M
. The resultant of all acting
forces is directed downward into the liquid and is described as surface tension in our
macro world. Thus, surface tension is caused by the cohesive intermolecular forces
of water acting on water molecules at the surface of liquid water.
The language of physics says that surface tension is a force related to a unit
length, Newton per meter, N/m. Usually, it is more practical and convenient to use
the surface pressure expressed as Pascals, Pa, where 1 Pa is one Newton per one
square m, 1 Pa = 1 N/m
2
. Since it is extremely small unit, we indicate the surface
pressure in hectopascals, 1 hPa = 100 Pa, or in kilopascals, 1 kPa = 1,000 Pa. When
we measure dried-out soils, we use units in megapascals, MPa = 10
6
Pa = 10
4
hPa.
The meteorologists are used to pressure units in millibars, where 1 mbar = 1 hPa.
The conversion of units is according to the following relations: 1 Pa = 1 N/
m
2
= 10
−5
bar being the equivalent of 0.00001 bar = 10.197 × 10
−6
at (technical atmo-
sphere) = 9.8692 × 10
−6
atm (atmosphere) = 7.5 × 10
−3
Torr (Torr = 1 mm of
Hg) = 1.45 × 10
−4
psi (pounds per square inch). Surface tension on the fl at plain
water level is the resultant of cohesion action of the water molecules located below
the water level. It is 7.28 × 10
−2
N/m or 72.8 dyn/cm at the temperature 20 °C.
Fig. 8.5
Simplifi ed model of
the intermolecular forces
acting upon the water
molecule on the plain
horizontal water surface and
upon water molecule in the
interior of water. The
molecule on the surface,
attracted in one-sided
direction downward into the
liquid, is observed as surface
tension on the macroscale