Biology Reference
In-Depth Information
pseudo-helical conformation through coordination to palladium
and chirality organization through NH (Ala)/Cl, NH (adjacent to
pyridine unit)/O (Ala), and NH (adjacent to pyridine unit of another
molecule)/O (Ala) intramolecular hydrogen bonds, in which two
ferrocene-dipeptide conjugates coordinate to palladium center
unsymmetrically (Figs. 3.11 and 3.12a). It should be noted that the
NH adjacent to the pyridyl moiety of one peptide chain participates
in the intramolecular hydrogen bonding with the CO of the Ala of the
same peptide chain to nucleate a
-turn-like structure. This chirality-
organized structure is in sharp contrast to the crystal structure of
g
,
in which an intermolecular hydrogen bonding network is observed
instead of the intramolecular hydrogen bonds to induce a helically
ordered molecular assembly (Fig. 3.9a) [10]. These findings suggest
that the complexation with PdCl
6
-turn-like
structural regulation of the dipeptide chain through intramolecular
hydrogen bonding in the crystal structure. Furthermore, each
molecule is connected through intermolecular hydrogen bonds
between the NH of the Ala and the CO adjacent to the ferrocene unit
(another molecule) to form a left-handed pseudo-helical molecular
arrangement (Fig. 3.13a). A good mirror-imaged chirality-organized
(MeCN)
induces the
g
2
2
O
O
Fe
Fe
N
H
N
H
Me
Me
N
N
O
O
O
O
Cl
Cl
N
N
H
H
N
Pd
N
N
Pd
N
H
H
N
N
Cl
Cl
O
O
O
O
N
N
Fe
Fe
N
H
N
O
Me
Me
O
H
{Fc(-L-Ala-D-Pro-NH-2-Py)
1
}
2
−
PdCl
2
9
{Fc(-D-Ala-L-Pro-NH-2-Py)
1
}
2
−
PdCl
2
10
Figure 3.11
The palladium complexes
with the ferrocene-
peptide bioconjugates bearing the dipeptide chains (-Ala-
Pro-NH-2-Py).
9
and
10



































































































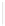

















Search WWH ::

Custom Search