Biology Reference
In-Depth Information
alanine and a sterically constrained proline as a well-known turn
inducer in proteins. The conformational enantiomers based on the
torsional twist about the Cp(centroid)-Fe-Cp(centroid) axis are
possible with the 1,1
′
-disubstituted ferrocene as shown in Fig. 3.3
[5,9]. The ferrocenoyl moiety of
-helical arrangement.
The incorporation of the dipeptide chains into the ferrocene scaffold
achieves the chirality-organized structure based on two rigid
interchain intramolecular hydrogen bonds, although a wide range of
relative orientations are possible depending on two rotatory Cp rings.
The crystal structure of
1
adopts the
P
composed of the corresponding D-dipeptide
chains (-D-Ala-D-Pro-OEt) confirms the
2
-helical arrangement of
the ferrocenoyl moiety (Fig. 3.2b) [8c]. The molecular structures of
1
M
are in an excellent mirror image relationship as shown in
Fig. 3.2, indicating the presence of conformational enantiomers (Fig.
3.3). As a result, the introduction of the chiral dipeptide chains into
the ferrocene induces the chirality organization by restriction of
the torsional twist through the interchain intramolecular hydrogen
bonds [8c]. Furthermore, a mutually opposite helically ordered
molecular arrangement with one turn of 14.70 Å and 14.95 Å pitch
height and 8.35 Å and 8.16 Å separation (Fe-Fe) between the closest
ferrocene units, respectively, is observed in the crystal packings of
1
and
2
(Fig. 3.4a and b), in which the ferrocene moieties arrange
in a herringbone motif to form the columns of the proline and ethyl
ester moieties. Protein folding is driven by not only the hydrogen
bonding but also the hydrophobic effect [1]. The dipeptide chains,
-Ala-Pro-OEt, are considered to induce such molecular aggregation
through a hydrogen bonding site (Ala) and a sterically constrained
hydrophobic moiety (Pro).
and
2
Fe
Fe
O
O
N
N
H
H
O
O
Me
Me
O
O
O
O
N
N
H
H
O
O
N
N
N
N
Me
Me
O
O
O
O
Fc(-L-Ala-L-Pro-OEt)
2
1
Fc(-D-Ala-D-Pro-OEt)
2
2
Figure 3.1
The ferrocene-peptide bioconjugates
1
and
2
bearing the
dipeptide chains (-Ala-Pro-OEt).






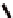
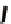


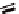
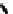























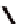



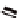


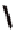



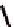

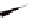
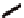







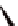


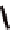














Search WWH ::

Custom Search