Biology Reference
In-Depth Information
When we started our own approach toward such appealing
structuresin2001,broaduseofknownpeptidenanotubeswas
invariably thwarted by their inherent insolubility [62]. We then
reasoned that if a cyclohexapeptide like cyclohexaglycine self-
assembled as tubes whilst assuming a
symmetric hexagone
conformation (for the sake of simplicity), each such bracelet-shaped
cyclopeptide would experience as many as 12 hydrogen bonds
withtwoneighbouringrings(Fig.2.6).Thisarrangementwould
result in six hydrogen bonds used to join two consecutive rings. The
corresponding rather huge stacking energy of 36 kcal/mol might
well be seldom responsible for the insolubility of the material since
the equilibrium is likely strongly biased towards the nanotube to the
detriment of the free constitutive cyclopeptides.
S
6
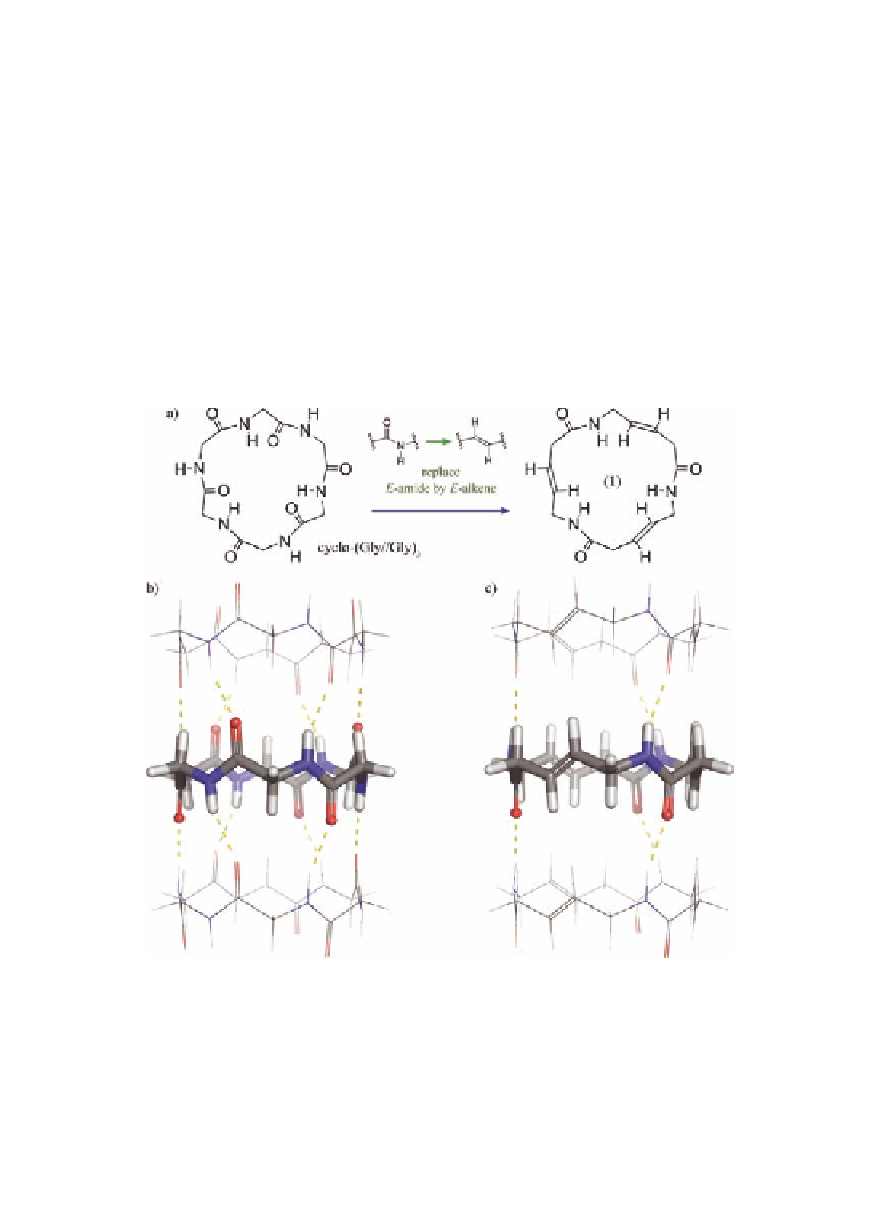
Figure 2.6
(a)Designoftarget(
) from cyclohexaglycine. Hypothetical
stacking of (b) cyclohexaglycine and (c) target (
1
1
) showing
the hydrogen bonds.
A straightforward way around the insolubility problem could
then be to simply reduce the number of hydrogen bonds involved
Search WWH ::

Custom Search