Biology Reference
In-Depth Information
13
C-DQF
DRAWS experiments constrained the peptide termini to a bilayer
structural model (Fig. 1.13A).
with the N-terminal lysine out-of-register, solid-state NMR
F} REDOR solid-state NMR
experiments established that the half of the N-terminal lysines
buried within the leaflet interface can be passivated by stoichio-
metric sequestering of trifluoroacetic acid (TFA) (Fig. 1.13B) [24].
13
C{
19
A
9.8Å
5.2Å
B
10Å
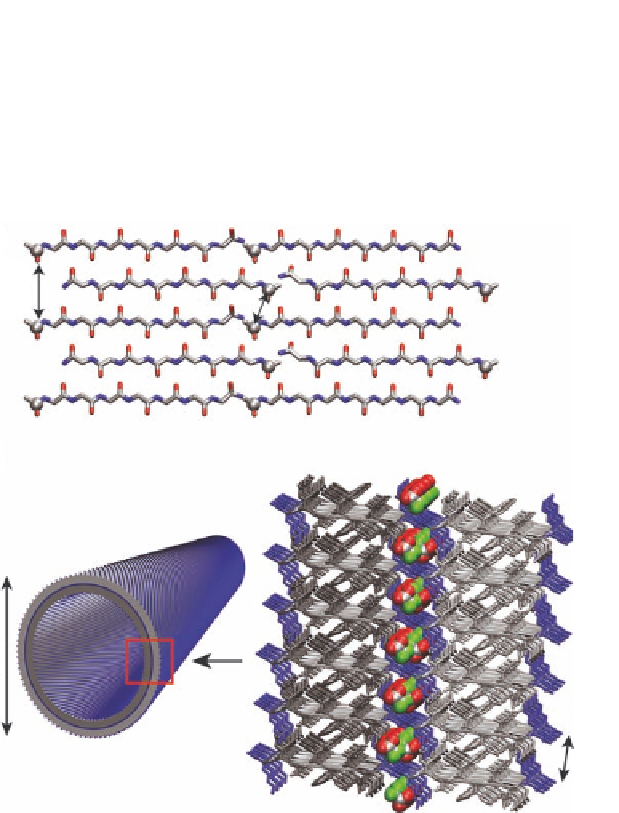
Figure 1.13
KLVFFAE and KLVFFAL bilayer models (
) showing positions
for the acetate carbonyl carbons (gray spheres). The anti-
parallel out-of-register peptide configuration places the
distance of the solvent-exposed acetates at 9.8 Å, which is
too far to be detected by solid-state NMR, and restricts the
measured
13
CO-
13
CO distance of 5.2 Å to the bilayer interface
[24]. (
A
) Bilayer model of tube wall composed of anti-parallel
out-of-register
B
β
-sheets. N-terminal lysines are colored blue
and hydrophobic residues, including the C-terminal leucine
or protonated glutamate, are colored gray. Gray shading
highlights the individual leaflets. The high density of positive
charge at the bilayer interface is passivated by TFA counter-
ions (space filling with fluorine - green, oxygen - red, and
carbon - gray).


Search WWH ::

Custom Search