Biology Reference
In-Depth Information
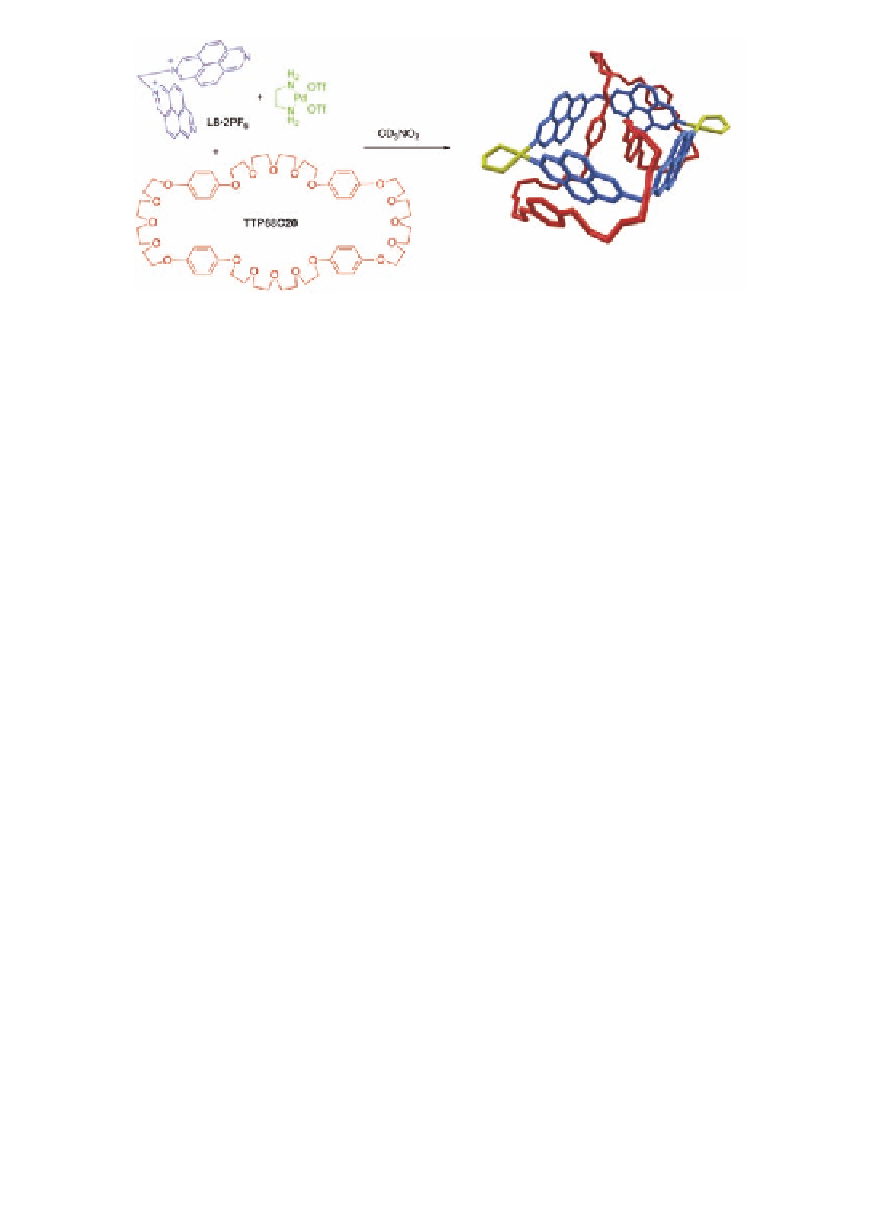
Scheme 11.6
Synthesis of a doubly interlocked [2]catenane and its crystal
structure.
The X-ray crystal study revealed a [2]catenane structure with
the metallocycle doubly encircled by the cyclophane
TPP68C20
(Scheme 11.6). Two of the dioxoaryl rings are inserted into the
metallocycle displaying a π
stacking arrangement of four aromatic
systems with a mean interplanar distance of 3.59 Å. The other two
donor rings are located outside of the metallocycle but also involved
in π
-
π
-
π
interactions.
11.7
Conclusions and Outlook
The success of the design and construction of molecular devices such
as switches, motors, muscles, machines, wires, storage-information
systems, etc., relies on chemical synthesis of the building blocks, their
assembly into supramolecular entities, and finally, their transfer
onto solid substrates.
During the last decades, the synthesis and self-assembly of
catenanes have been one of the most rapidly developing areas of
supramolecular chemistry. In this context, catenanes represent an
interesting class of candidates for the construction of molecular
devices because their dynamic structure can be adequately
switched when an external stimulus is applied. For instance, a solid-
state catenane-based molecular switch has been fabricated and
demonstrated to work between the “on” and “off” states. Although
the difference between the “on” and “of” states in this design is still
much too small (in terms of resistance) to be useful for logic circuits,
the switches could be useful for memory devices [37].
Search WWH ::

Custom Search