Biology Reference
In-Depth Information
to check the regioselectivity with acyclic guests such as
S1a,b
and
S2a,b
. Thus, the addition of acyclic guests to a solution of
M6a
·6NO
3
O resulted in the partial or total reorganization
of the “incorrect” metallocycle
and
M
′
6a
·6NO
in D
3
2
M
′
6a
·6NO
to yield the 1:2 inclusion
3
. Obviously, the mechanism
requires, as in the case of catenanes, the dissociation of the N atoms
from the Pd
complexes derived from
M6a
·6NO
3
II
centers. In contrast to catenanes, in these cases the
inclusion equilibrium between the complexed species and their
separated components is fast in
1
H NMR time scale so only averaged
signals are detected.
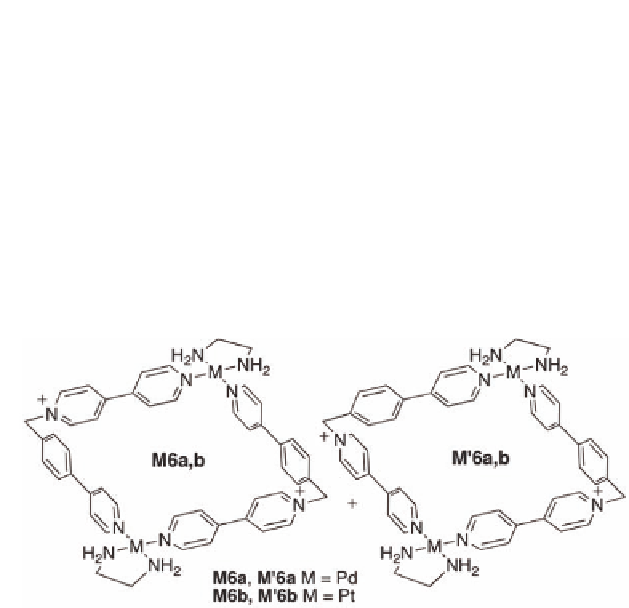
Figure 11.12
Regioisomeric metalocycles
, which result from
two possible orientations of the starting materials.
M6
and
M'6
, and
other guests allowed us to draw some conclusions. First, cyclic guests
(in acetonitrile solution) seem to favor higher regioselectivities than
their acyclic counterparts (e.g.,
The comparison of the results obtained from
S1a,b
,
S2a,b
BPP34C10
and
S1a,b
). Assuming a
similar π-donor capacity for the dioxoaryl rings of
BPP34C10
and
S1a,b
and almost identical entropic costs for all the self-assembly
processes, then the number and strength of the π-interactions
with the metallocycles determine the regioselectivity. Thus, the
ratio of π-interaction energies between
M
′
6a
(
BPP34C10
)
/
2
M6a
(
BPP34C10
)
is smaller than those of the inclusion complexes
2
M
′
6a
resulting in a higher regioselectivity for the
former. Second, naphthalenic substrates achieve higher selectivities
than phenylenic derivatives owing to their better π-donor character.
Finally, the presence of the polyether chains in the acyclic guest (e.g.,
(
S1a
)
/
M6a
(
S1a
)
2
2

Search WWH ::

Custom Search