Biology Reference
In-Depth Information
-1
-1
-1
M
,
K
= 915
±
35 M
, and
K
= 1292
±
47 M
, respectively). The
a
a
slightly higher
K
value for
BPP34C10
pseudorotaxane compared
a
to those of
presumably reflects the greater
flexibility of this cyclophane to maximize the [C-H
…
O] hydrogen
bonds.
DB24C8
and
DN38C10
The crystal structure of the 1:1 complex between
L5
·2PF
and
6
1
DB24C8
H NMR data, that the complex
is stabilized by [C-H
…
O] hydrogen bonds between methylene
protons and the oxygen atoms of the polyether chain in addition with
[C-H
…
O] interactions between the
revealed, in agreement with
α
-CH bipyridinium hydrogens
and these oxygen atoms.
has adopted a rare C-shaped
conformation in contrast to a the S-shaped one observed in 1,2-bis-
(pyridinium)ethane systems [32]. A π
DB24C8
stacking interaction is also
observed between one of the aromatic rings of
-
π
and one
of the bipyridine systems. The angle between the planes is 5
DB24C8
°
, and
the distance between the centroid of the
DB24C8
ring involved in
the π
stacking interaction and the centroid of the bypyridinium
system is 3.55 Å (Fig. 11.10a).
-
π
(a)
(b)
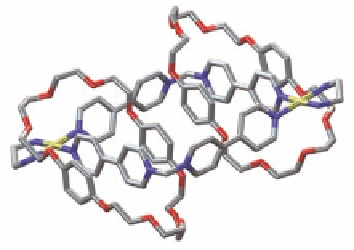
Figure 11.10
(a) Crystal structure of pseudorotaxane complex between
L5
showing [C-H
…
O] hydrogen bonds
·2PF
and
DB24C8
6
stacking interaction. (b) Crystal structure of the [3]
catenane (
and
π−π
). Solvent molecules, counter
ions, and hydrogen atoms have been omitted for clarity.
Color labeling scheme as in Fig. 11.5.
BPP3410
)
-(
M5a
2
Therefore, our strategy involves the threading of the crown
ethers
BPP34C10
,
DB24C8
, and
DN38C10
with the ligand
L5
·2PF
6
followed by closing of the resulting pseudorotaxanes with metal
centers owning two
cis
labile ligands affording in that manner the



Search WWH ::

Custom Search