Biology Reference
In-Depth Information
stereoisomers can probably be ascribed to the fact that, assuming a
similar insertion mode for 1,3-DHN, only three [C-H
1] interactions
(at positions C-4, C-7, and C-8) can be established between 1,3-DHN
and the quinoline systems in a
syn
conformation.
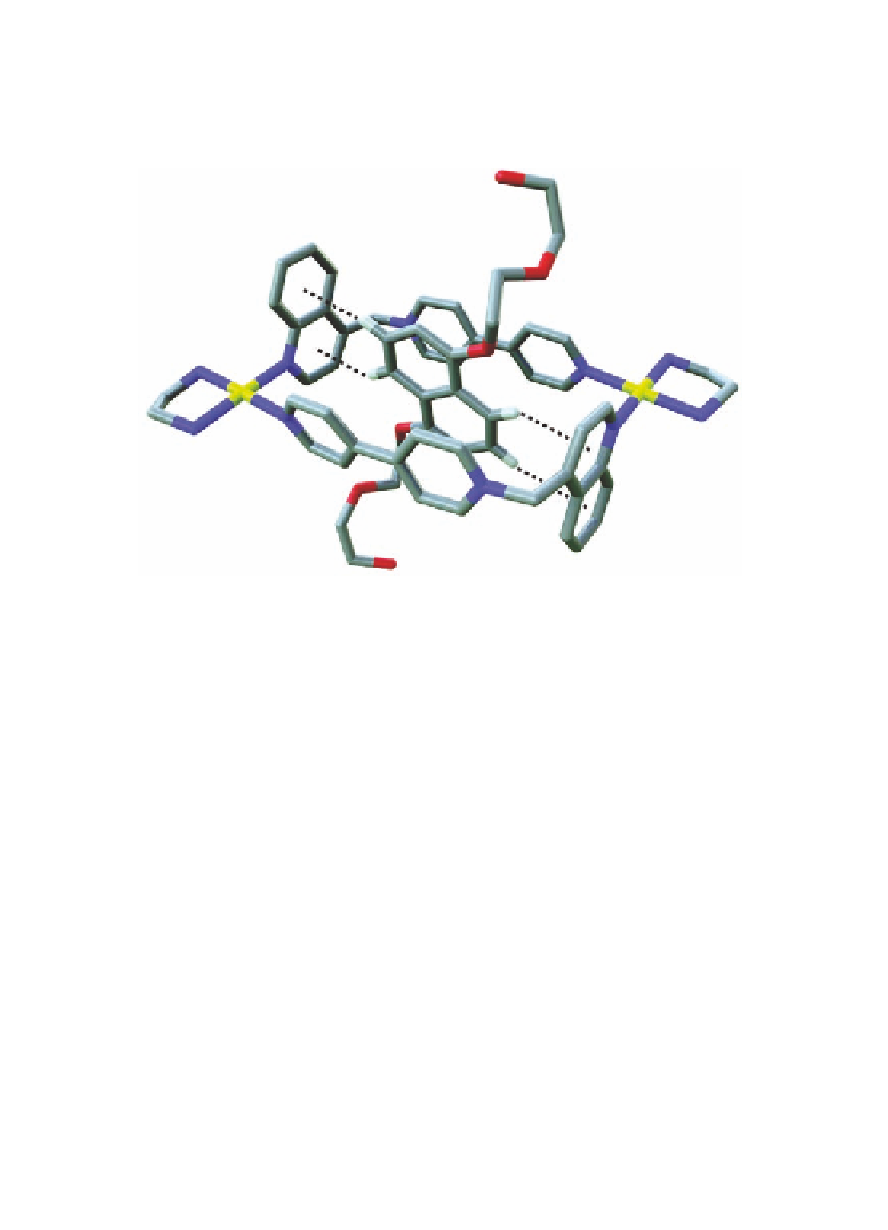
Figure 11.9
Crystal structure of
S2b
⊂
M7a
·6NO
showing the four
3
[C-H
…
] interactions. Solvent molecules and hydrogen
atoms (except those involved in hydrogen bonding) have
been omitted for clarity. Color labeling scheme as in Fig.
11.5.
π
11.5
Catenanes
An [
interlocked
macrocycles [29]. These molecules, apart from their intrinsic beauty,
display interesting physical properties, and some can be considered
as molecular machines or motors [9-12]. The synthesis of catenanes
has been one of the topics where supramolecular chemistry has
obtained numerous successes. The use of self-assembly processes
guided by noncovalent interactions facilitates the preorganization
of the molecular components prior to the cyclization reactions.
Following our self-assembly strategy, the synthesis of catenanes is
obvious. Simply, the use of cyclic substrates such as the
n
]catenane is a molecular system composed of
n
DB24C8
,
DN38C10
, and
BPP34C10
(Fig. 11.4) instead of acyclic ones in
Search WWH ::

Custom Search