Biology Reference
In-Depth Information
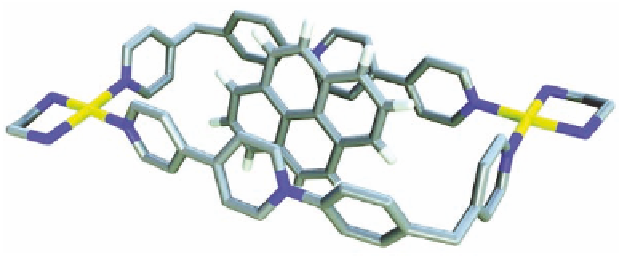
between the centroid defined by the atoms of the long side of the
rectangle and the plane of pyrene is 3.40 Å, exactly the optimum
calculated distance for systems held by face-to-face π-stacking
interactions. The long side of the metallocycle measures 14.75 Å,
slightly longer than the calculated one. The plane containing the
pyrene molecule is inclined by 70
with respect to the equatorial
plane of the metallocycle defined by its four corners, while the axis
defined by pyrene protons H2 and H7 forms an angle of 30
°
with
the same plane. Another consequence is the decrease on the torsion
angle in the bipyridinium moiety from 31
°
°
to 25
°
as a result of the
π
-
π stacking interactions.
Figure 11.6
View
of
the
crystal
structure
of
inclusion
complex
pyrene
. Color-labeling scheme as in Fig. 11.5.
Hydrogen atoms from the metallocycle, solvent molecules,
and counter ions have been omitted for clarity.
⊂
M4a
·6NO
3
11.4.2
Inclusion Complexes with 1:2 Stoichiometry
As described earlier, expansion of one of the metallocycle sides
allows the complexation of larger substrates. Conversely, extension
of the other side makes the cavity size large enough (ca. 10.4 Å) to
receive two molecules of substrate (Fig. 11.3). Thus, addition of one
equiv. of (en)Pd(NO
(10 mM) at room
temperature gives rise to the formation of metallocycle
)
to a D
O solution of
L5
·2NO
3
2
2
3
.
The addition of two equiv. of hydroquinone to a 5 mM solution
of metallocycle
M5a
·8NO
3
in water produced a pale red coloration.
This is related to the presence of an intermolecular charge transfer
absorption band centered at 439 nm [27]. The crystal structure
M5a
·8NO
3


Search WWH ::

Custom Search