Biology Reference
In-Depth Information
Moreover, additional stabilization can be achieved by [C-H
O]
hydrogen bonding between oxygen atoms of the polyether chains
and hydrogens at the
α
or β-bipyridinium positions.
Figure 11.4
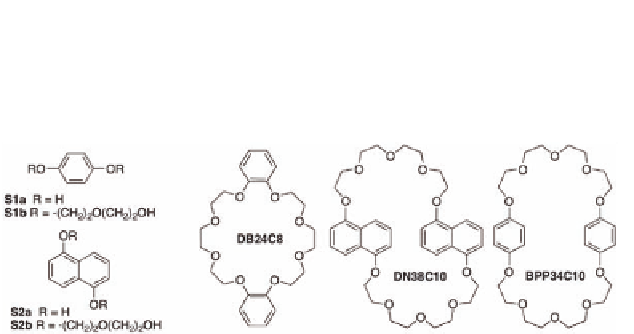
Examples of aromatic substrates.
Solutions of equimolar amounts of diol
S2b
and metallocycle
in nitromethane were red-colored as a result of the
expected charge-transfer derived from the π
M3b
·5PF
6
1
H
NMR spectrum of the mixture showed broad signals that indicate an
equilibrium in the solution close to coalescence. When the sample was
cooled to 250 K the signals appeared well defined, so that the spectral
changes originated by the formation of the supramolecular complex
can be interpreted. Each of the
-
π
interaction. The
1
splits
in two upon formation of the inclusion complex. Moreover, the same
signal splitting is observed for the resonances of the naphthalene
moiety. This fact can be attributed to the inclusion of
H NMR signals of
M3b
·5PF
6
S2b
inside the
cavity, lowering the symmetry of the receptor from
C
symmetry in
s
S2b
⊂
M3b
M3b
. The nonequivalent protons H-4
and H-8 of the naphthalene moiety interact by means of [C-H
·5PF
to
C
in
·5PF
6
1
6
π]
bonds with the short sides of the metallocycle, as demonstrated
by the pronounced upfield shifts of their signals up to 5 ppm (Fig.
11.5a). The reverse downfield shifts of the accepting aromatic ring
proton signals are also an evidence for this interaction. Another
consequence of the
symmetry of the inclusion complex is that two
different isomers may exist in solution depending on the position of
the guest within the cavity. These isomers are enantiomers, and their
existence is consistent with the observed
C
1
1
H NMR spectrum. Similar
results were obtained for metallocycles
M2
. An acridine derivative
II
of the Pd
metallocycle
M2
allowed us to characterize the inclusion
complexes with
S1a
and
S2b
by X-ray crystallography.

Search WWH ::

Custom Search