Biology Reference
In-Depth Information
10.6
Porphyrin-Based Metallacyclic
Supramolecules for Molecular Recognition
and Sensing
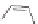
The rich photochemistry of porphyrins can be utilized to construct
metallacyclic supramolecules, in which significant change in
absorption as well as luminescence properties can take place upon
guest encapsulation. In the zinc(II) metallaporphyrin cycle,
41,
reported by Hunter
et al.
, a functionalized macrocyclic cavity is created
through a Lewis acid
base interaction between the Zn(II)-porphyrin
and pyridine [90]. The intramolecular hydrogen bonding between
the central pyridine and amide N
−
−
H ensures the approximate right
angle in the supramolecular assembly. The porphyrin cycle was able
to encapsulate appropriate terephthalamide derivatives (
)
with strong hydrogen bonding provided by the amide functionality
of the ligand (see Scheme 10.5). The association constants (
43
and
44
K
=
a
−1
1400 and >1400 M
for
43
and
44
, respectively) were determined
by titration in CDCl
H NMR spectroscopy. The
terephthalic ester and bulky groups around the carbonyl moieties
showed weak interaction. However, isophthalic acid derivatives
bound very weakly since the distance between the carbonyl groups
is too short for the simultaneous formation of the hydrogen bonds
with amide groups at both the ends of the cavity.
monitored by
1
3
R
R
O
O
N
N
N
N
N
Z
n
R
H
Z
n
R
N
N
N
N
N
N
R'
R
R
R'
O
R'
O
O
N
N
O
NH
N
O
41
N
O
O
O
N
HN
N
R'
R
R
N
N
N
N
N
H
N
N
N
N
Z
n
N
R
Z
n
R
N
N
O
O
R
42
R
O NH(C
6
H
13
)
O R'
O N(C
6
H
13
)
2
R =
=
Guests:
C
6
H
13
(C
6
H
13
)HN
O
(C
6
H
13
)
2
N
O
R'
O
43
44
Scheme 10.5















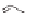















































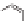
















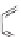








































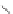



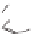









































































































Search WWH ::

Custom Search