Biology Reference
In-Depth Information
nm with quantum yield of 0.05 in CH
CN solution. The small Stoke
3
1
π→π
*
fluorescence. The X-ray
crystal structures of the inclusion complexes show the presence
of
shift indicates that the emission is
π−π
interactions between the guests and the bipy and edge-to-
face or C
−
−π
bonding between the guests and the anthracenyl
backbones of the host. Comparison of the structures of the inclusion
complexes suggests the manifestation of an induced-fit mechanism
in the complexation. The emission of rectangle
H
was quenched
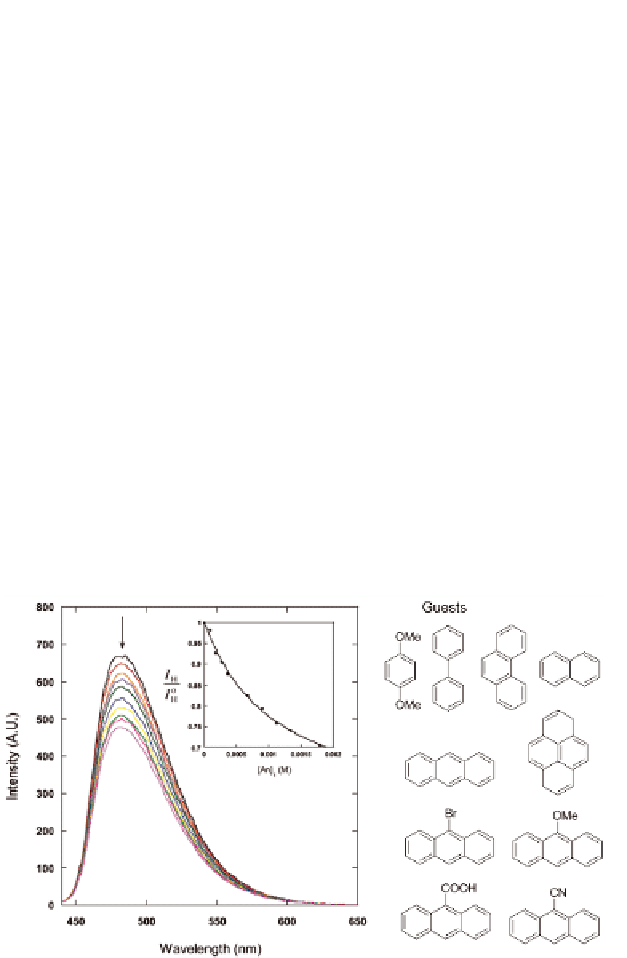
in the presence of a series of aromatic guests (see Fig. 10.5). The
corresponding binding constants determined from emission titration
are in close agreement with the binding constant obtained from
32
1
H
NMR titration. This leads to the conclusion that the quenching occurs
owing to the binding of the guest with the Au-metallacyclic host, i.e.
it is not due to the dynamic quenching between the host and guest
molecules. In general, the sparingly soluble polycyclic anthracene,
substituted anthracene, phenanthroline, and pyrene bind more
strongly to
,
than the more soluble 1,4-dimethoxybenzene, biphenyl,
and naphthalene. The solvophobic effect, ion
32
dipole interaction and
edge-to-face interaction are attributed to the effective formation of
host
−
−
guest inclusion complexes between
32
and aromatic guests.
Figure 10.5
Emission spectral change upon addition of anthracene to
a CH
. Excitation wavelength = 420 nm;
excitation and emission slit width = 10 nm. Inset shows
the emission titration curve and the least-squares fit. The
emission intensity of the host is monitored at 480 nm.
Reproduced with permission from Ref. [82].
CN solution of
32
3

Search WWH ::

Custom Search