Biology Reference
In-Depth Information
nm to 338 and 420 nm, respectively, as well as a slow decay in the
intensity of the bands with the time. Likewise, the emissions also
show red shift of the bands at 499 and 476
−
534 nm upon photolysis
(see Fig. 10.1). The appearance of an isosbestic point at 436 nm in
the absorption spectra indicates a clear photochemical conversion.
The red shifts in the absorption and emission spectra are believed
to occur owing to the breaking of the triangle and releases the
steric strain associated with the structure and this increases the
conjugation of the aromatic system to form a polymerized product
(see Scheme 10.1).
Figure 10.1
(A) Absorption spectra of
9b
in 1,2-DCE at 293 K as a function
l
= 0, 1, 2, 3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, and 14 h. (B) Normalized emission spectra
of
of photolysis time (
= 313 nm).
t
ex
9b
in 1,2-DCE at 293 K as a function of photolysis time
= 0, 1, 2, 3, 4, 5, 6, and 14 h. Reproduced
with permission from Ref. [41].
(
l
= 313 nm).
t
ex
RO
CO
C
O
OC
OC
Re
N
N
Br
Re
CO
Br
CO
N
N
N
OR
313 nm
OR
OR
OR
RO
RO
RO
O
C
N
N
RO
Br
Re
Re
OC
OC
N
N
CO
RO
N
OC
CO
Br
R = C
6
H
13
,
a
; C
12
H
25
,
b
OC
OR
N
N
Re
CO
n
OC
Br
OR
9
10
Scheme 10.1
features a
broad band centered at 383 nm and a shoulder at 422 nm [41]. The
The absorption spectrum of the dinuclear complex
11












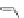







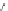

























































































































Search WWH ::

Custom Search