Biology Reference
In-Depth Information
only fibers were detected. So indeed, and much like the F-F dyad,
histidines side chains distributed on both the top and bottom
β
-sheet
faces can contribute to the formation of Zn(II)-containing tubes,
and argues that the Zn(II)-imidazole coordination stabilizes facial
complementarity and extended
β
-sheet lamination (Fig. 1.8B).
Figure 1.8
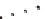
Incorporation of intra-sheet interactions that have resulted
in extended
β
-sheet lamination: (
A
) KLVFFAE displaying
) HHQALVFFA + Zn
2+
displaying His-
Zn-His interaction that bridges laminates, (
the Phe-Phe dyad, (
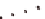
B
) ccQALVFFA
displaying the cytosine i-motif interaction across laminate.
(
C
-sheet fiber has two distinct solvent-
exposed surfaces: one composed of side chains (gray) and
one composed of peptide N (blue) and C (red) termini.
The termini surfaces (blue/red) form laminate grooves.
The side-chain surface (gray) displays the β-sheet pleats.
Transformation of an amyloid fiber into a nanotube by
increasing the number of laminates; the N−C termini surface
area (blue/red) increases while fusion into a tube covers the
pleat surface (gray/yellow) except at the very ends of the
nanotube.
D
) The amyloid
β
Based on this success, and the knowledge of the 10 Å lamination
spacing between the sheets approximating that of a DNA duplex, the

















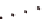

































































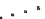



















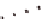





















































































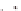






















































































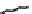











































































































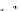












































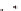







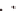





















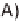














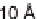



















Search WWH ::

Custom Search