Biology Reference
In-Depth Information
3
-1
(
K
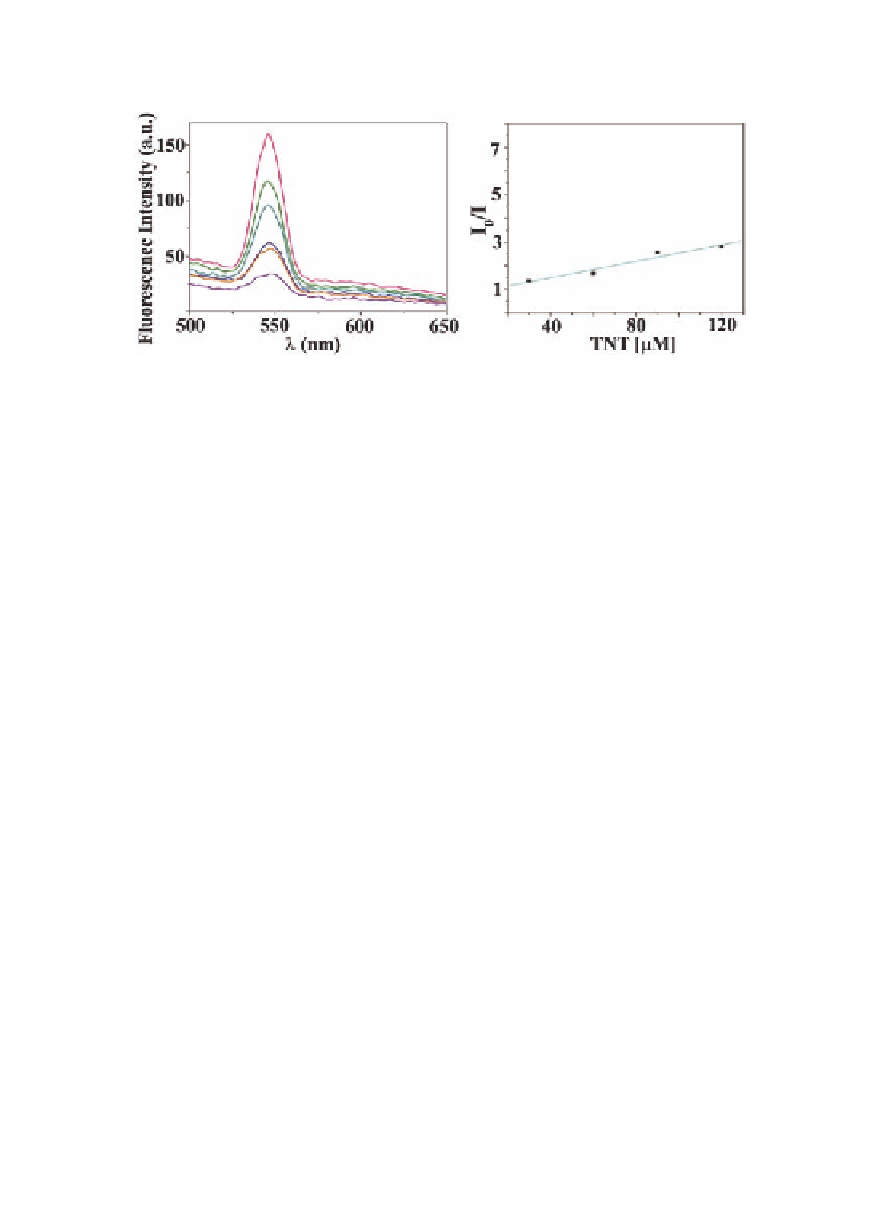
) was determined to be (Fig. 9.21) 19.6 × 10
M
in CH
Cl
-DMF
SV
2
2
(4:1).
Figure 9.21
Fluorescence quenching of
17
by TNT (left) and Stern-
Volmer plot (right).
The fluorescence intensity in the solution state of both
21
and
23
underwent quenching upon gradual addition of TNT (2,4,6-
trinitrotoluene) solution. When a 1.5 × 10
in
DMF was titrated with a TNT solution in methanol, the fluorescence
intensity gradually decayed as shown in Fig. 9.22. The mechanism
of fluorescence quenching could be due to the formation of charge-
transfer complex between the excited state of
-6
M solution of
21
π
-electron-rich cage
21
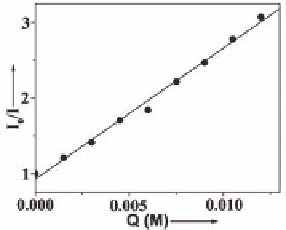
and an electron-deficient oxidizer TNT. The Stern-Volmer plot
(Fig. 9.22) gave a quenching constant of
-1
K
= 173.3
±
5.46 M
. The
SV
high quenching constant in case of
21
was probably due the presence
of electron-donating -CH
, which makes the cage a better host for
3
electron-deficient TNT.
Figure 9.22
Fluorescence quenching of
21
by TNT (left) and Stern-
Volmer plot (right).

Search WWH ::

Custom Search