Biology Reference
In-Depth Information
Table 9.1
Photophysical data of the supramolecular assemblies.
Complex
Absorbance
l
max
(nm)
Fluorescence
l
max
(nm)
Ф
F
18
283, 365
425
0.04
21
421, 398, 309
430, 460
0.35
23
430, 383, 270
472
0.48
9.4
Nitroaromatics Detection via Fluorescence
Quenching
Detection of trace analyte is a central challenge in the field of
chemical sensors. Nowadays owing to security reasons more and
more attention is directed toward sensing and detecting of explosive
compounds. Generally, explosives comprise an intimate mixture of
oxidizing and reducing agents that can undergo a highly exothermic
reaction and decomposes to yield gaseous products. Nitroaromatics
are very commonly used components in explosive mixtures. The
detection of nitroaromatics can be done by exploiting their highly
electron-deficient properties, which lead to their excellent electron-
withdrawing capabilities. Owing to the presence of highly electron-
withdrawing nitro groups on the aromatic ring it lowers the energy of
the empty π
*
orbitals, thus making it highly electron accepting. This
property can be used in the fluorescence quenching of fluorophores
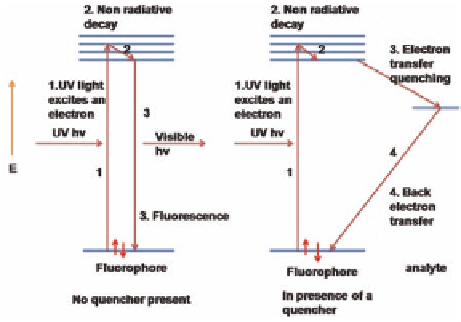
[37-39]. Fluorescence is usually achieved by an electron-transfer
donor-acceptor mechanism (Scheme 9.12).
Scheme 9.12
Mechanism of electron transfer fluorescence quenching.







Search WWH ::

Custom Search