Biology Reference
In-Depth Information
Molecular Prisms From 4,4
¢
,4
¢¢
-Tris[Ethynyl-
Trans
-
Pt(PEt
3
)
2
(NO
3
)]Triphenylethane
9.3.3
9.3.3.1 Synthesis of the Pt
3
Acceptor
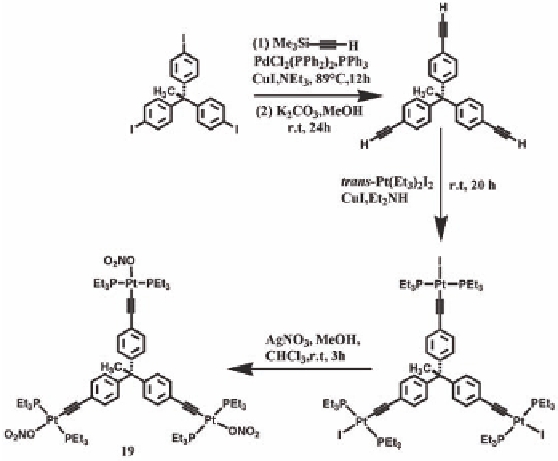
To expand this methodology of using [2 + 3] self-assembly of two
components, another novel pyramidal organometallic Pt
acceptor
3
)]triphenylethane was
synthesized. The iodide analogue of the carbon-centered new
tripodal acceptor
4,4
¢
,4
¢¢
-tris[ethynyl-
trans
-Pt(PEt
)
(NO
3
2
3
was prepared from 1,1,1-tris(4-iodophenyl)
ethane, which was prepared according to the literature procedure.
Coupling of trimethylsilylacetylene (Me
19
SiCCH) with 1,1,1-tris(4-
iodophenyl)ethane followed by the desilylation gave the ethynyl
incorporated compound 1,1,1-tris(4-ethynylphenyl)ethane (Scheme
9.10). Treatment of 1,1,1-tris(4-ethynylphenyl)ethane with four
equivalents of
3
trans
-(PEt
)
Pti
in the presence of CuI catalyst in
3
2
2
dry Et
NH medium at room temperature overnight produced 1,1,1-
2
tris[4-(
I)ethynylphenyl]ethane, which was separated
by column chromatography and isolated in yellow microcrystalline
form. The tri-nitrate derivative
trans
-Pt(PEt
)
3
2
19
was synthesized from 1,1,1-tris[4-
(
I)ethynylphenyl]ethane upon treatment with three
equivalents of AgNO
trans
-Pt(PEt
)
3
2
at room temperature in 88% isolated yield.
3
Scheme 9.10
Synthesis of the Pt
acceptor
19
.
3


Search WWH ::

Custom Search