Biology Reference
In-Depth Information
9.2.2.3 Cation Sensing
A fluorescent sensor usually comprises of a light-emitting group
(fluorophore), which is covalently linked to a receptor specific for
a particular ion/substrate [25]. In case of fluorescent sensors, the
enhancement or quenching of fluorescence intensity can be used as
an efficient tool for the detection of various ions and chemicals in
the solution. Fluorescent sensors are very useful for the selective
detection of ions and chemical entities in current scenario [14].
Sensor efficiency requires that the ion-receptor interactions modify
the fluorescence of the light-emitting unit. Hence, the fluorescence
can be switched on and of depending on the nature of the ion/
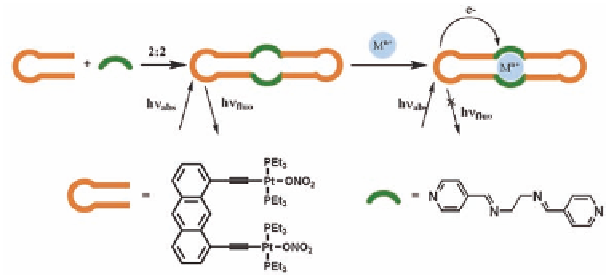
substrate-receptor interactions [26]. Scheme 9.5 illustrates the basic
principle for such a molecular switch.
Scheme 9.5
Mechanism of a molecular switch functions as a biosensor.
Owing to its strong luminescence and chemical stability, the
anthracene unit has been widely used in the design of fluorescent
sensors. It has also been well established that Pt-ethynyl compounds
show luminescence behavior [27,28]. These properties have been
exploited in the design and synthesis of the fluorescent rectangles
8-11
showed emission band at around 460
nm upon excitation at 400 nm in methanol solution (Fig. 9.5).
In this chapter the deviations in the fluorescence properties of
the rectangle
. The complexes
8-11
on binding with various metal ions are discussed.
Our rectangle design
(11)
has an anthracene-based fluorophore
required for a fluorescent sensor and a N
(11)
pocket containing four
imine nitrogen atoms inside the molecule, which can coordinate
with the metal ion of appropriate size. The fluorescence intensity
4


Search WWH ::

Custom Search