Biology Reference
In-Depth Information
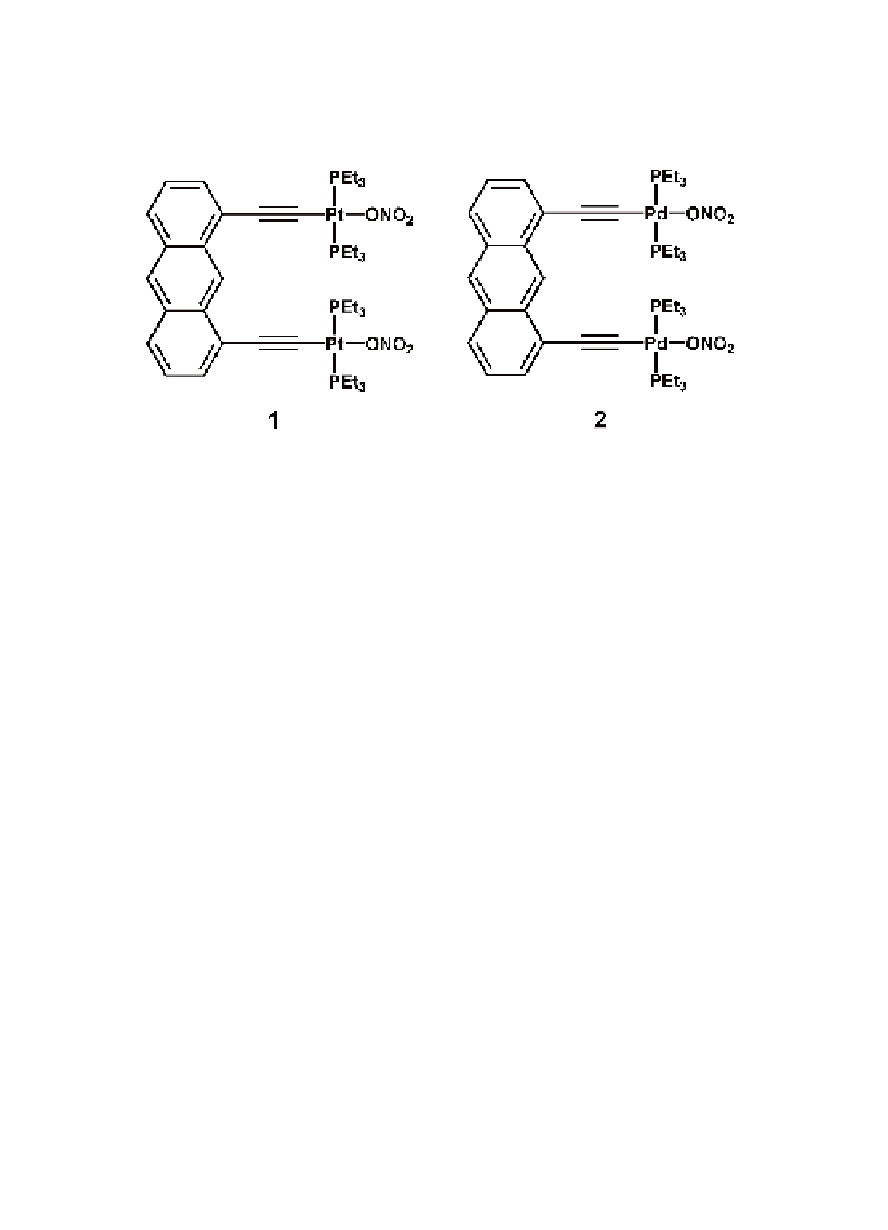
To extend the Stang's methodology of [2 + 2] molecular rectangle
synthesis [20,1] and to explore the possibility of designing fluorescent
rectangles, we have prepared both Pt
molecular “clips”
with the incorporation of ethynyl functionality (Fig. 9.1) [22,23].
II
and Pd
II
Figure 9.1
Structures of molecular “clips” with the incorporation of
ethynyl functionality.
9.2.1
Synthesis and Characterization of the
Organometallic Pt
II
2
(1)/Pd
II
2
(2) Molecular “Clips”
1,8-Dichloroanthracene was treated with (trimethylsilyl)acetylide
magnesium-bromide (Me
SiCCMgBr) which was prepared by
reacting ethylmagnesiumbromide with 1,8-dichloroanthracene in
THF under nitrogen atmosphere. Hydrolysis of the resulting product
1,8-bis(trimethylsilylethynyl)anthracene in methanol using K
3
CO
2
3
produced 1,8-diethynylanthracene. This 1,8-diethynylanthracene
was then further reacted with 3.5 equivalents of
trans
-MI
(PEt
)
2
3
2
[M = Pd or Pt] in presence of CuI(
1
)/CuCl(
2
) as catalyst to give 1,8-
I]anthracene, which was isolated in pure
form by column chromatography. 1,8-Bis[
bis[ethynyl-
trans
-M(PEt
)
3
2
trans
-Pt(PEt
)
I(ethynyl)]
3
2
anthracene was treated with 2.1 equivalents of AgNO
in a mixture
3
of chloroform and methanol to obtain
1
in 90% isolated yield
(Scheme 9.3).
1
31
P},
electro-spray ionization mass spectrometry (ESI-MS), and finally
by X-ray diffraction method. The molecular structures of these
“clips” were conclusively assigned by X-ray single-crystal structure
analysis. Figure 9.2 represents the views of the molecular structures
The “clips”
1-2
were fully characterized by NMR {
H and
Search WWH ::

Custom Search