Biology Reference
In-Depth Information
tricarboxyamides (BTAs) readily self-assemble in organic solvents
into columnar stacks via a combination of
stacking interactions
and threefold hydrogen bonding between the amide groups. By
attaching three porphyrins to this core, we expected a further
strengthening of the intermolecular interactions in a columnar stack
because of the large
π
-
π
π
-surfaces of the porphyrins. Indeed, porphyrin
trimer
(Fig. 8.7A-B) was found to self-assemble strongly in
organic solvents such as chloroform, hexane, and toluene [15], and
at concentrations above
4
2 mM in chloroform it was even able to
gelate the solvent. Scanning tunnelling microscopy measurements
of the trimers at the graphite/1-phenyloctane interface showed the
presence of columnar stacks of which the internal structure could be
molecularly resolved.
∼
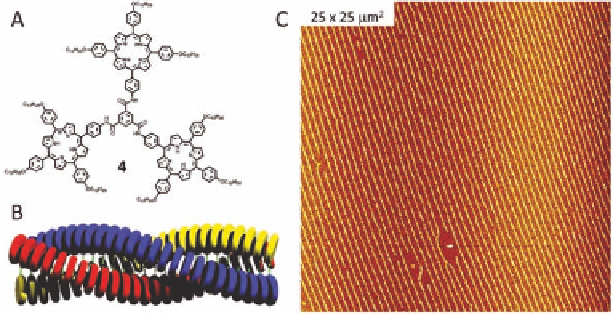
Figure 8.7
(A) Molecular structure of porphyrin trimer
4
. (B) Cartoon
representing the proposed self-assembly of
into a columnar
stack. (C) AFM image of the extended line patterns obtained
when a chloroform solution of
4
4
is evaporated on mica.
-6
M) of the trimers in
chloroform was deposited by dropcasting onto a mica surface and the
solvent allowed to evaporate, surprisingly no rings were observed,
but instead huge domains of highly defined line patterns (Fig. 8.7C)
were observed. The majority of the surface was covered with these
patterns, and uniform domains as large as a square millimetre were
observed. The lines were all 4.5
When a highly diluted solution (<10
±
0.4 nm high, which indicates that
they are composed of single molecule thick columnar stacks of
.
The most striking feature of the patterns, however, was the high
4


Search WWH ::

Custom Search