Biology Reference
In-Depth Information
m
exhibited anisotropic fluorescence. Only on the hydrophilic carbon-
coated glass surface this anisotropy could already be observed
without an additional annealing step, and it indicates a high degree
of molecular ordering within the rings on this surface. Porphyrin
dodecamer
microscopy experiments revealed that only rings smaller than
∼
5
µ
was found to form highly defined and monodisperse
rings on both the hydrophilic and hydrophobic carbon-coated glass
surfaces, indicating even stronger intermolecular interactions
between the molecules of this compound [13].
3
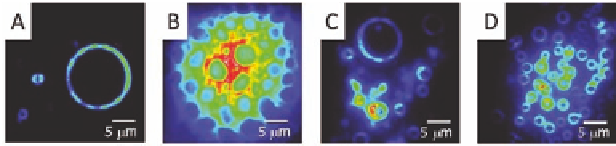
Figure 8.6
Fluorescence microscopy images of rings formed after
the evaporation of a chloroform solution of
on (A) a
hydrophobic carbon-coated copper grid, (B) untreated glass,
(C) glass with a hydrophobic carbon coating, and (D) glass
with a hydrophilic carbon coating. Copyright Wiley-VCH
Verlag GmbH & Co. KGaA. Reproduced with permission.
2
on hydrophilic
carbon-coated glass could be assessed qualitatively, by evaluating
the fluorescence intensity as a function of the centre angle
The degree of polarization in the rings of
2
(Fig.
8.4C). The intensity curve shows a clear modulation with a period
of 180
f
, which is a reflection of the transmission dipole moments of
the porphyrins at different locations in the ring. For a ring in which
the porphyrin molecules are perfectly organized according to the
representation in Fig. 8.5, the fluorescence intensity should drop
to zero in the minima. The fact that this is not the case might point
at imperfect ordering, but it can also indicate a possible transport
of excitation energy along the ring. To investigate this possibility,
rings were excited by a laser, which was tightly focused down to the
diffraction limit (
°
m in diameter). This excitation did not result
in the emergence of fluorescence emission in the rest of the ring,
which discards the possibility of energy transport along the ring and
suggests that the fluorescence at the intensity minima is caused by
a less perfect ordering of the porphyrins, which might be caused by
∼
1
µ


Search WWH ::

Custom Search