Biology Reference
In-Depth Information
chromophores to displace significantly and spend more time in the
uncoupled excited state.
2x2
4A
RO
O
P
O
O
O
O
O
O
N
O
O
O
O
O
S
N
O
O
O
S
O
N
O
O
S
O
O
O
N
O
O
O
O
O
O
O
O
N
N
O
O
O
O
O
O
O
S
O
S
N
S
O
O
O
S
O O
S
N
O
O
O
O
O
O
O
N
O
O
N
O
O
O
N
O
O
O
N
O
O
O
O
O
O
RO
rotation
P
O
P
O
O
RO
O
O
O
O
O
O
O
O
N
O
O
N
O
O
O
N
O O
O
O
O
O
S
N
S
O
O
O
N
O
O
O
S
O
O
O
N
O
O
O
O
O
O
O
O
O
N
S
O
O
O
O
S
S
N
N
O
O
O
O
S
O
S
O
N
O
O
O
O
O
O
O
O
O
O
N
O
O
O
N
O
O
O
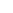
BzO
OH
breathing
RO
P
O
O
O
O
O
P
O
O
RO
O
O
O
O
O
O
O
O
O
O
O
O
O
N
N
O
O
O
O
N
O
N
O
N
O
O
N
O
O
O
O
O
O
O
O
O
N
O
O
S
S
O
S
O
O
O
S
N
O
S
O
O
O
O
O
O
O
S
O
O
O
O
N
N
O
O
O
O
N
O
O
O
O
O
N
N
N
O
O
S
O
O
O
O
O
O
O
O
S
O
O
O
O
N
O
O
O
O
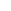
P
O
RO
O
O
BzO
O
O
O
N
O
OH
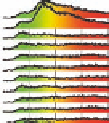
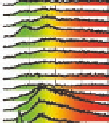
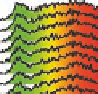
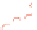
Figure 5.24
Portions of spectral trajectories for a single linear tetramer
4A
(left) and a single concatenated tetramer
2
x
2
(right).
Emission from
often displays dynamic spectral switching
attributed to photoinduced folding and unfolding while
4A
tends to show “steady-state” emission due to its concatenated
structure. Thirty-seven frames at 2 s integration/frame =
∼
2
x
2
74 s total elapsed time.
The compelling spectral trajectory data reveal that structurally
constrained cyclic compounds have dramatically different dynamics
when compared with the series of linear foldamers, and strongly
support that photoinduced displacements (folding/unfolding) create
dynamic spectral switching, both disrupting and reforming perylene
π
−
2 Å
can sufficiently quench excited-state delocalization or annihilate
excitons, which explains why the cyclic compounds also display
substantial monomer-like emission that is even observed in very
long perylene stacks [71,72]. Following photoinduced unfolding, the
cyclic compounds are much more likely to return to the folded state
more quickly than we can detect and therefore spectral trajectories
-stacks. Chromophore separation or translation of only 1



































































































































































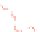





























































































































































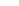






















































































Search WWH ::

Custom Search