Biology Reference
In-Depth Information
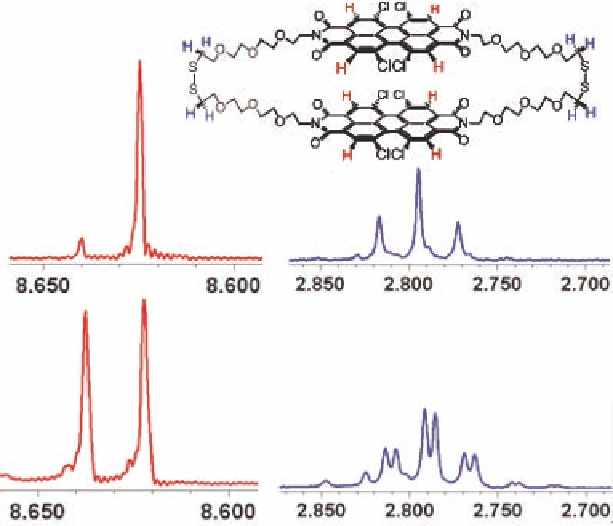
Figure 5.20
NMR monitors isomerization reaction. Top:
exhibits one
peak for all eight identical aromatic protons (left, red) and
only one triplet for protons adjacent to the disulfide bonds
(right, blue). Bottom:
2B
reaching a
50/50 diastereomeric mixture in about 24 h. The mixture
then exhibits two equally integrated perylene peaks and two
equal triplets for protons adjacent to the disulfide. Solvent:
CDCl
2B
interconverts to
2K
.
3
To determine if the two compounds were truly interconverted
diastereomers, normal-phase HPLC was used to separate and purify
the suspected isomers. The compounds separated surprisingly well,
with heterochiral cyclic dimer
2K
eluting before the homochiral cyclic
because of the more steric and soluble heterostructure
(Fig. 5.21). The separate fractions were collected, and immediately
characterized by mass spectrometry (ESI); each compound exhibited
identical mass and isotopic distributions consistent with the cyclic
dimer containing eight chlorine atoms (Fig. 5.21).
dimer
2B


Search WWH ::

Custom Search