Biology Reference
In-Depth Information
5.5.4
Molecular Codes Control Reactions
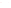
Scheme 5.9 outlines how phosphoramidite coupling chemistry
complies with the shape-change enabled molecular codes, which
manifests different reaction yields because similarly coded molecules
effectively reduce the coupling activation entropy penalty. Reacting
the bifunctionalized planar monomer
1C
with various twisted
monomers
1D
−
1H
in separate reactions yielded bichromophoric
cyclic compounds
, which were isolated in all cases in addition
to other linear products. However, the reaction yield of the desired
binary cyclic compounds abated with increasing twist angle of the
starting monomers
2D
−
2H
. Scheme 5.9 summarizes each reaction
yield, revealing the existence of molecular encoding effects during
reactions.
1D
−
1H
N
N
O
O
P
OH
O
O
P
O
O
O
O
O
O
O
O
O
N
O
N
O
O O
N
O O
N
O
O
O
O
N
O
O O
N
N
O
O
O
=
Cl
Cl
O
R
1
O
R
4
O
Cl
Cl
Br
Br
Br
O
R
2
O
O
N
O
R
3
O
N
O
O
N
O
O
N
O
O
N
O
O
N
O
N
O
O
N
O
O
N
O
O
O
N
O
a, b
R
1
+
R
4
R
1
Monomers 1D 1E 1F 1G 1H
R
4
R
2
R
2
R
3
R
3
2G
25
o
6%
0.28
-
2H
37
o
4%
0.06
1.57
Cyclic Dimers
∆θ
(deg)
yield (%)
∆δ
(ppm)
r
obs
2D
0
o
27%
0.65
0.73
2E
14
o
27%
0.50
0.88
2F
23
o
6%
0.35
1.17
O
N
O
O
N
O
O
N
O
O
N
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
P
O
1C
OH
O
O
2D
-
2H
N
N O
1D-1H
N
Scheme 5.9
The planar bisphosphoramidite
1C
was reacted separately
with various twisted monomers (
1D
−
1H
) to form cyclic
dimers
increases, reaction yields diminish
owing to the deteriorating compatible assembly codes.
Concomitantly,
2D
−
2H
[9]. As
∆
q
π
∆
d
-stack induced upfield chemical shift (
)
decreases and the vibronic peak ratio (
) increases, thus
providing reliable metrics of code compatibility. a: CH
r
obs
Cl
,
2
2
N
-phenyl-imidazolium triflate. b: 0.2 M I
(CH
Cl
/Py/H
O
2
2
2
2
1:3:1).
For the reaction between planar precursors, stronger attractions
drive self-assembled stack formation, reducing the activation
entropy for coupling and favorably orienting the reaction centers


















































































































































































































































































































Search WWH ::

Custom Search