Biology Reference
In-Depth Information
concatenated dimer
−
dimer
2
x
2
than monocyclic dimer
2A
, which in
turn should be larger than the monocyclic monomer ring
1
. Indeed,
this was what we observed, thus confirming that dimer
−
dimer
2
x
2
and monocyclic dimer
2A
exist as cyclic
folded
structures. In the
concatenated rings
, there is little free rotation of one ring with
respect to the other because
2
x
2
interactions effectively dock the
perylene units on top of each other.
π−π
S
S
O
20x10
3
a
O
1546.6
O
O
O
O
1545.5
1569.8
O
N
O
N
O
O
15
1544.5
1567.7
772.1
10
O
O
N
O
N
O
1380.6
803.9
832.1
936.2
5
O
O
1320.3
1484.0
O
O
1215.3
O
O
S
S
0
600
800
1000
1200
1400
1600
1800
2A
Mass (m/z)
12x10
3
10
8
6
4
2
0
S
S
b
O
O
3113.1
S
S
O
O
O
O
O
O
O
O
O
O
3128.0
N
N
O
O
O
O
N
O
O
N
O
O
1546.9
1484.2
O
O
O
O
O
O
N
O
N
N
O
N
1383.4
O
O
772.9
O
O
3090.7
O
O
O
1569.1
O
O
1320.9
O
S
S
O
O
S
S
2
x
2
1000
1500
2000
2500
3000
3500
Mass (m/z)
8x10
3
O
O
N
O
c
772.39
772.39
O
6
O
773.39
S
4
S
774.39
775.39
O
O
2
O
N
O
O
0
600
800
1000
1200
1400
1600
1800
Mass (m/z)
1
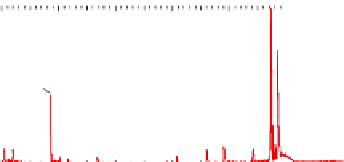
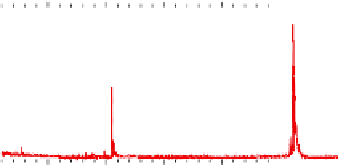
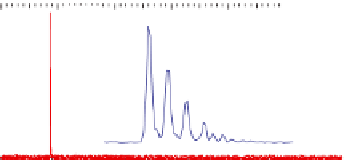
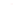
Figure 5.16
MALDI-TOF mass spectra for (a) macrocyclic dimer
2
, (b)
dimer
.
Inset in (c) is an expansion of the peak around 772.39 mass
units revealing the isotope distribution.
−
dimer catenane
2
x
2
, and (c) macrocyclic monomer
1
Compared to the dimer rings (
2A
,
2
x
2
), proton chemical shifts
on the TEG chain of
undergo upfield shifts because they bridge
over the perylene segment that imparts an aromatic ring current
shielding effect to the TEG chain. For example, the differences of
1



















































































































































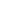





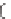














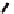





























































































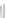






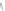



































Search WWH ::

Custom Search