Biology Reference
In-Depth Information
influence the reaction pathways of cyclization and concatenation
of perylene derivatives. No template is needed as the reactants
undergo the assembly process by self-templating. In such a reaction,
molecular self-assembly serves as the precursor for cyclization
and concatenation reactions. The resulting molecular assembly
has an optimal configuration that resembles to the transition-state
activation complex, which promotes certain reaction pathways and
discourages others. In essence, molecular self-assembly, which serves
to preorganize reactants, effectively lowers the entropic barrier from
normally chaotic reactants into an organized transition state.
To illustrate the relationship between dynamic self-assembly and
reaction products, we employ
SAc [49], which
undergoes appreciable self-organization, forming a twisted
1I
or
AcS
−
perylene
−
π
-cofacial
conformation above its critical concentration region,
C
∼
0.1
−
1.0
c
mM in CHCl
and CH
Cl
solvents (Scheme 5.6). Accordingly, a series
3
2
2
of CH
Cl
solutions of
1I
under air at concentrations immediately
2
2
, namely 2.58, 0.58, and 0.1, respectively,
was investigated. Deacetylation of
above or below the
C
c
(-Ac) with several drops of
2 M NaOMe/MeOH formed reactive intermediate counterparts
of
1I
in solution (solution color changes from red to blue), which
subsequently yielded various cyclic compounds linked by disulfide
bonds upon air oxidation (TLC Rf: 0.24
1I
/MeOH: 20/1).
Of particular interest was that only two major products were
obtained, dimer-based monocyclic disulfide
−
0.42, CH
Cl
2
2
2A
and its catenane
2
x
2
(H) neutralization of
the reactions. HPLC analysis for the reaction of 0.58 mM reactant
1I
(Scheme 5.6) after Amberlite 1R-120
*
∼
∼
yielded
39% for
2A
and
36% for
2
x
2
, a total isolated yield of
∼
75%, and other unidentified products including trace amounts of
polymers.
ϕ
ι
S
S
S
S
S
S
AcS
O
O
O
AcS
η
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
γ
O
S
S
O
O
O
O
O
O
O
φ
O
O
O
δ
χ
ε
O
O
N
O
N
N
N
O
O
N
O
O
O
O
N
N
O
O
O
N
O
N
O
O
N
O
O
O
O
O
O
α
k
1
β
k
2
+
2
2
NaOMe/MeOH
CH
2
Cl
2
NaOMe/MeOH
CH
2
Cl
2
Air
O
O
N
O
O
O
O
O
O
N
O
O
O
O
O
N
O
Air
N
N
O
O
O
O
N
N
O
N
O
N
N
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
S
S
O
O
O
O
O
O
S
S
S
S
S
S
AcS
2A
2A
2
x
2
AcS
1
I
1
I
Scheme 5.6
Dynamic self-assemblies of
1I
direct the formation of
cyclic dimer (
), which self-organizes with another two
monomers and undergoes3 concatenation to yield
2A
2
x
2
.








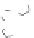












































































































































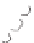




















































































































































































































Search WWH ::

Custom Search