Biology Reference
In-Depth Information
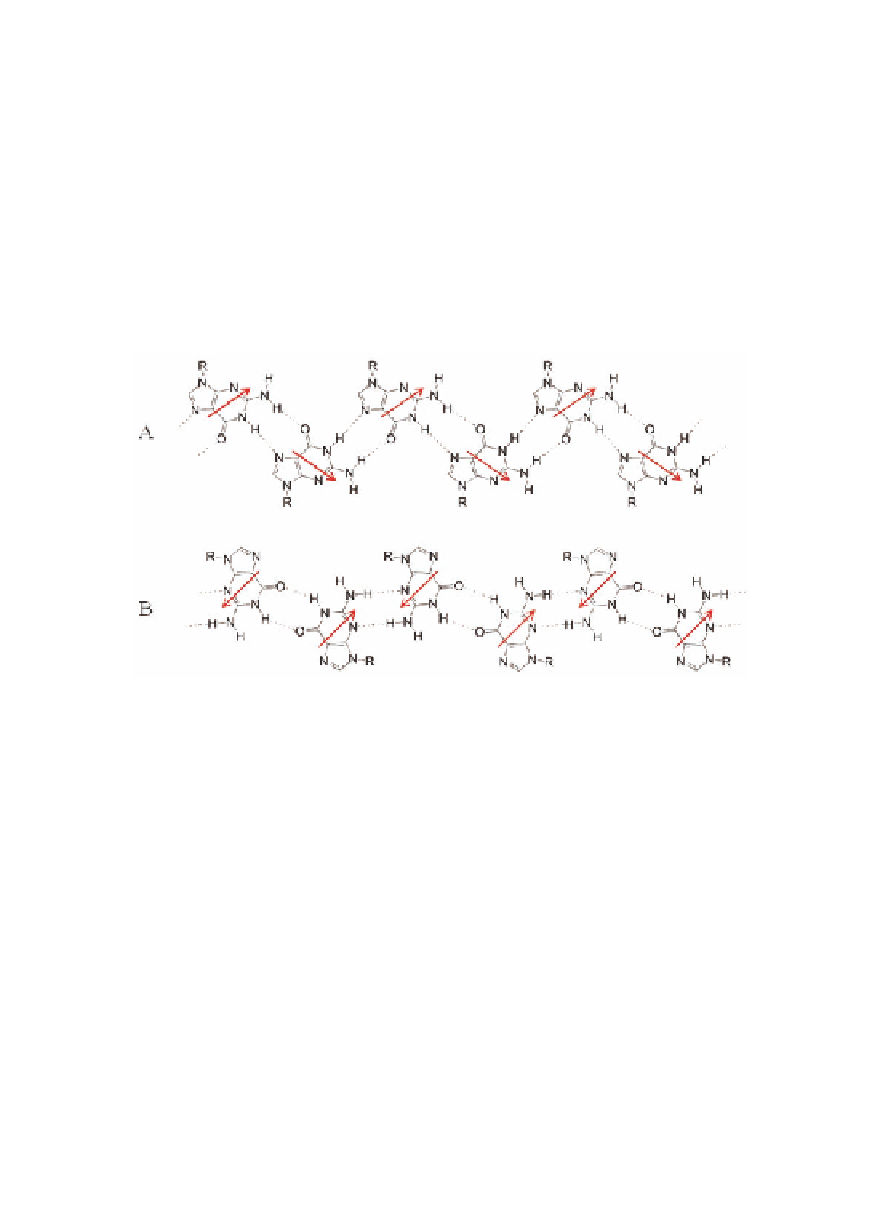
the solid state and is detected in solution in (anhydrous) chloroform
soon after dissolving the polycrystalline powder, is held together by
an intramolecular N
hydrogen bond network. In
solution, the ribbon A may slowly undergo a structural transition
toward a thermodynamically more stable form (ribbon B, Fig. 4.12B)
characterized by N
2
H-O
6
and N
1
H-N
7
1
6
2
3
intermolecular cyclic hydrogen
bonds: this is normally observed for dialkanoyl derivatives of type
1a
H-O
and N
H-N
. Upon adsorption at the solid-liquid interface, the guanosine
supramolecular ribbons B undergo a back rearrangement to the
A-type ribbons. In both ribbon-like polymers the glycosydic bonds
adopt an
anti
conformation.
Figure 4.12
H-bond pattern of ribbon-like assemblies. A) Ribbon A, solid
state; B) ribbon B, in solution. Arrows indicate molecular
dipoles.
In the absence of templating cations, the formation of ribbon-
like polymers seems to be a quite general behavior for LGs, as long as
the molecules can adopt an
anti
conformation around the glycosidic
bond.
It should be noted that the two ribbons posses a different
symmetry. As a consequence, while in ribbon B (solution) molecular
dipoles cancel each other, ribbon A structure (solid state) has a
permanent dipole moment and, remarkably, in the crystal structure
ribbons are arranged in a parallel fashion, thus making polar the
whole bulk [25b].
Search WWH ::

Custom Search