Biology Reference
In-Depth Information
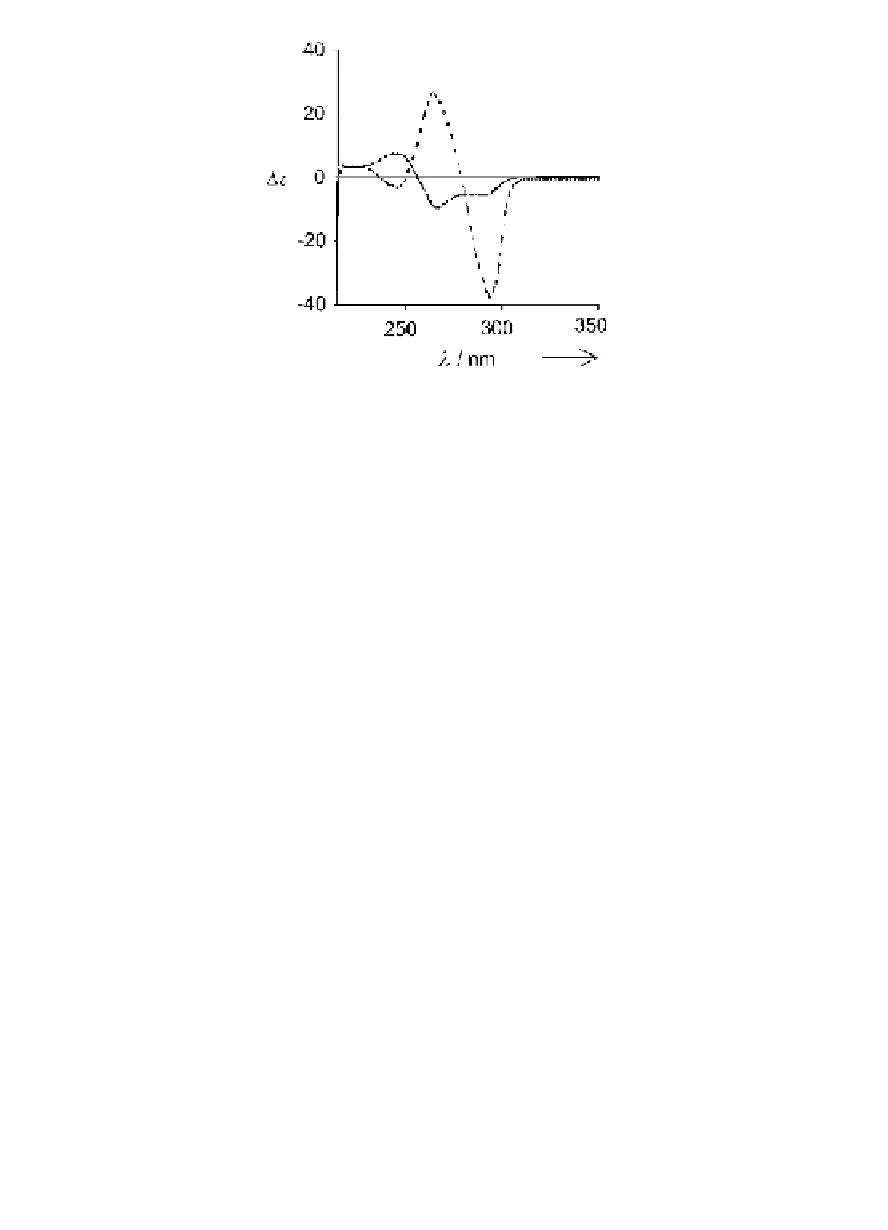
Figure 4.9
Comparison between CD spectra of
C
4
- (solid line) and
8
.
M
+
(Data from
D
4
-symmetric (dashed line) octamers LG
references [9,34]).
4.3
Self-Assembly in the Absence of Metal Ions
Before describing the typical structures that LGs form in organic
solvents in the absence of templating cations, it is important to
mention that, in some instances, LGs can form isolated G-quartets
even in the absence of metal ions. This is observed when bulky
substituent (e.g., SMe or Br groups) are introduced at the 8 position
of the nucleobase [20,22]. Such behavior can be explained as the
consequence of a strong conformational preference for the
syn
isomer, imposed by the bulky substituent, that forces the exocyclic
amino group on top of the sugar ring, thus shielding both N
3
and
2
one of the N
H protons from hydrogen bonding (see below). The
observation by
15
spectra of
G-quartet formation in the absence of metal ions for two lipophilic
deoxyguanosine derivatives devoid of bulky substituents at the 8
position is a further example that challenges the formerly accepted
dogma that quartet formation requires metal ions [21].
When a hydroxy substituent is formally introduced at the 8
position of the nucleobase, a new, completely different self-assembly
pattern is observed. The preferred tautomeric form of the five-
membered ring in 8-oxoguanosine is lactamic (
N solid-state NMR refocused
inadequate
, Chart 4.2) and this
modified base possesses a rich sequence of hydrogen bond donor and
3
Search WWH ::

Custom Search