Biology Reference
In-Depth Information
Thus, like crown ethers, LGs can perform as ionophores but, in
this latter case, the actual ionophoric species is triggered by the ion
itself, via ion-dipole interactions, and is not a preformed covalent
structure. While the ability to form the octameric complex is quite
general, the formation of the
-polymer depends on the
structure of the guanosine derivative: deoxy-derivatives
pseudo
1a
normally
give rise to the
pseudo
-polymeric structure, derivatives of the
1b
or
1c
type, on the contrary, usually form octamers only. In addition, the
formation of the
-polymer strongly depends on the nature
of the counter ion, being favored by the best coordinating anions
(e.g., picrate, or PF
pseudo
6
-
). Cations able to promote octamer formation
are typically alkali metals, but also earth alkali or lanthanide ions
[10] have been reported. As far as cation selectivity is concerned,
our LGs show a slight preference for K
+
+
+
over Na
and Cs
. Noticeably,
derivatives
show a very strong affinity for alkali metal ions:
in some cases during standard chromatography over silica gel
these molecules do extract Na
1b
+
from the stationary phase and are
recovered as octameric complexes.
While we were publishing our first results on LGs, Jeff Davis
(University of Maryland) published similar experiments with
lipophilic isoguanosines [11]. A fruitful cooperation soon started,
which led first to the determination of the solution structure of the
octamer, the first detectable intermediate in the cation-directed
self-assembly of our LGs, by NMR spectroscopy [12]. Its structure
turned out to be similar to those of telomere models in which the
G-rich oligonucleotides are assembled into inter- or intramolecular
tetramers, which are composed of stacks of G-quartets [13]. This
octameric assembly is very robust and its stability is impressive for a
noncovalent assembly. The
1
H-NMR spectra, essentially temperature
independent over more than 100°C, show two sets of signals in a 1:1
ratio corresponding each to nucleosides with different glycosydic
conformation (
syn
-like and
anti
-like, Fig. 4.5).
H
O
O
N
H
N
H
2
N
N
N
X
N
RO
N
N
NH
2
RO
N
X
O
O
RO
RO
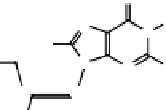
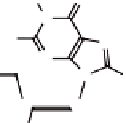
Figure 4.5
The
anti
(left) and
syn
(right) conformations of a guanosine
derivative.










Search WWH ::

Custom Search