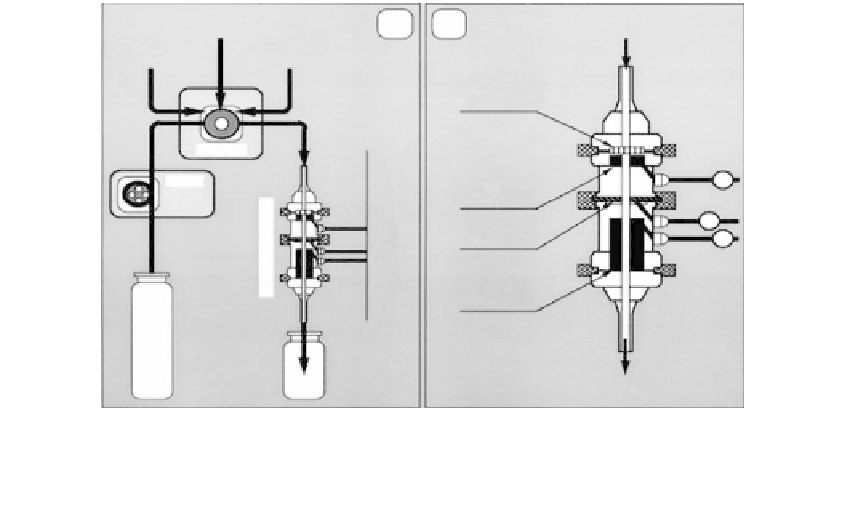
Biomedical Engineering Reference
In-Depth Information
E. coli
containing sample
Conjugate
solution
(a)
(b)
Inlet
Peroxide
solution
Immunofiltration
membrane
Injector
Pump
W
Working
electrode
R
Reference
electrode
C
Counter
electrode
Outlet
Waste
FIGURE 22.5
a) Flow-injection assay system; (b) immunofiltration sensor design.
determination in clinical biochemistry. This seems to be dependent on the material used for
blood collection. When hemolysis occurs during blood sampling, it decreases the reliability
of many results considerably. The use of partially or completely hemolyzed serum is
unavoidable, however, especially during field testing. Hemolysis can result from improper
drawing and handling of specimens and can also occur during centrifugation, separation
procedures, and dry freezing.
Hemolysis leads to interference by intracellular constituents in reactions of the assay.
The response of the device with hemolyzed samples can be seen Figure 22.6.
Experiments were performed under the same operating conditions as discussed earlier
using HRP-labeled conjugate [13]. It is obvious that the difference between the signals
obtained for negative and positive samples is insignificant, and they tend to overlap
beyond the operating dilutions shown. All efforts of trying to record a response at
dilutions other than that shown in Figure 22.6 have been failed due to the high degree of
noise and instability of the device with the hemolyzed samples. One reason is that the
broken erythrocytes have peroxidases that interfere with HRP. Since anodic oxidation of
hydrogen peroxide in the substrate is utilized for the amperometric detection of anti-
Hanta virus antibodies in blood samples, the presence of broken erythrocytes yield over-
lapping signals. Therefore, any chemical that can react with hydrogen peroxide will
interfere with the accuracy of the test. Hemolysis of red blood cells implies the presence of
debris (e.g., cell membranes), protein, peroxidases, and other potential interferents from
cells. A comparison of the response obtained for hemolyzed and nonhemolyzed negative
control samples is shown in [13]. It can be observed that in hemolyzed samples there is an
increase in response with increasing concentration of the sample; this is due to the bind-
ing of contents of the blood on to the surface of carbon particles and also on to the surface
of working, reference, and counter electrodes. However, we have not yet discovered
which compounds present in the hemolyzed blood are responsible for the nonspecific sig-
nals. Also exposing an electrode poised at
100 mV vs. Ag/AgCl to biological fluids will
produce a large background signal due to oxidation of endogenous sample components
[23]. Hence, optimization of the analysis requires large dilution of serum to avoid elec-
trode fouling and minimize the effect due to the presence of electro active species in
hemolyzed blood samples.