Biomedical Engineering Reference
In-Depth Information
diagnostics that also includes Group A Strep, RSV and Flu A
B, which is the first differen-
tiated test for Influenza A and Influenza B.
Now, there is a rapid
, in vitro
assay that detects and differentiates Influenza A and B viral
antigens in less than 15 min. BD Directigen™ Flu A
B is the first rapid assay on the
market that distinguishes Influenza A viral antigens from those of Influenza B.
Differentiation of Influenza A and B can help physicians select appropriate, cost-effective
treatment. Directigen™ Flu A
B offers a differentiated result, high accuracy, 15-min time-
to-results, easy workflow, and ability to test a wide range of specimen types.
Directigen™ Flu A
B Rapid Test Kit
Various specimen types include nasopharyngeal wash, nasopharyngeal aspirate, nasopha-
ryngeal swab, lower nasal swab, throat swab, and bronchoalveolar lavage. The
Directigen™ Flu A
B rapid test is the latest addition to the BD line of rapid respiratory
diagnostics that also includes RSV and Group A Streptococcus. Sensitivity and specificity
are its chief characterstics.
Influenza A: sensitivity of 95.7% and specificity of 91.4% for nasopharyngeal
aspirates and
●
Influenza B: sensitivity of 87.5% and specificity of 98.1% for nasopharyngeal
aspirates.
●
21.3.1.2
B from QUIDEL Company
Influenza is a highly contagious viral infection. There are three types of Influenza virus: A,
B, and C. Type A virus are most prevalent and are associated with the most serious epi-
demics. Type B virus produce a disease that is generally milder than that caused by A.
Type C virus have never been associated with a large epidemic of human disease. Both
type A and B virus can circulate simultaneously, but usually, one type is dominant during
a given season. This test is specific to Influenza type A and B (Figure 21.3). The QuickVue
Influenza A
The QuickVue Influenza A
B test allows for the rapid and qualitative detection of Influenza type A and
type B antigens directly from nasal swab, nasal wash, and nasal aspirate specimens. The
test is intended as an aid in the rapid differential diagnosis of acute Influenza type A and
type B virus infections [126].
The QuickVue Influenza test detects Influenza type A and B viral antigens in three easy
steps from a nasal swab or nasal-aspirate, or nasal-wash specimen. Test results are avail-
able in 10 min or less. The easy-to-use and easy-to-read kit can be stored at room temper-
ature. No instrumentation is required. The 10-test kit is conveniently packaged for
single-use testing and disposal. The 25-test kit offers bulk packaging that is ideal for batch
testing. Both the 10-test and 25-test kits include everything to perform the test including
positive and negative controls. Three types of results can be obtained.
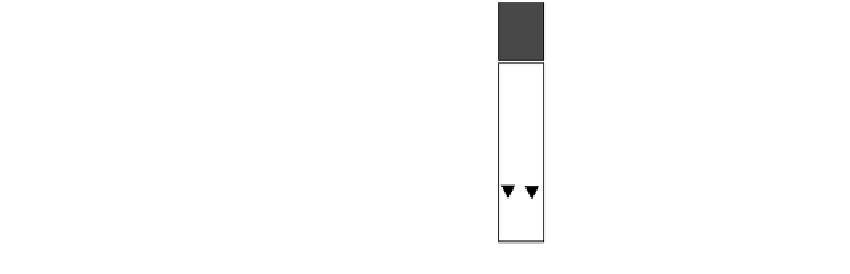
FIGURE 21.3
Results obtained with QuickVue Influenza A
B test. (From
www.quidel.com/Products/productdisp.php?prod=101&sec
tion
Positive
Negative
Invalid
pro. Accessed December 18, 2004.)