Biomedical Engineering Reference
In-Depth Information
20.1
Immunoglobulins and Immunoglobulin E
Antibodies are glycoprotein molecules which are produced by plasma cells in response to
an immunogen (1). They belong to a class of spherical proteins called globulins and are
hence known as immunoglobulins (Igs). Their primary function is to mediate the host
immune response by binding to antigens.
Immunoglobulins have been divided, on the basis of physical and functional properties,
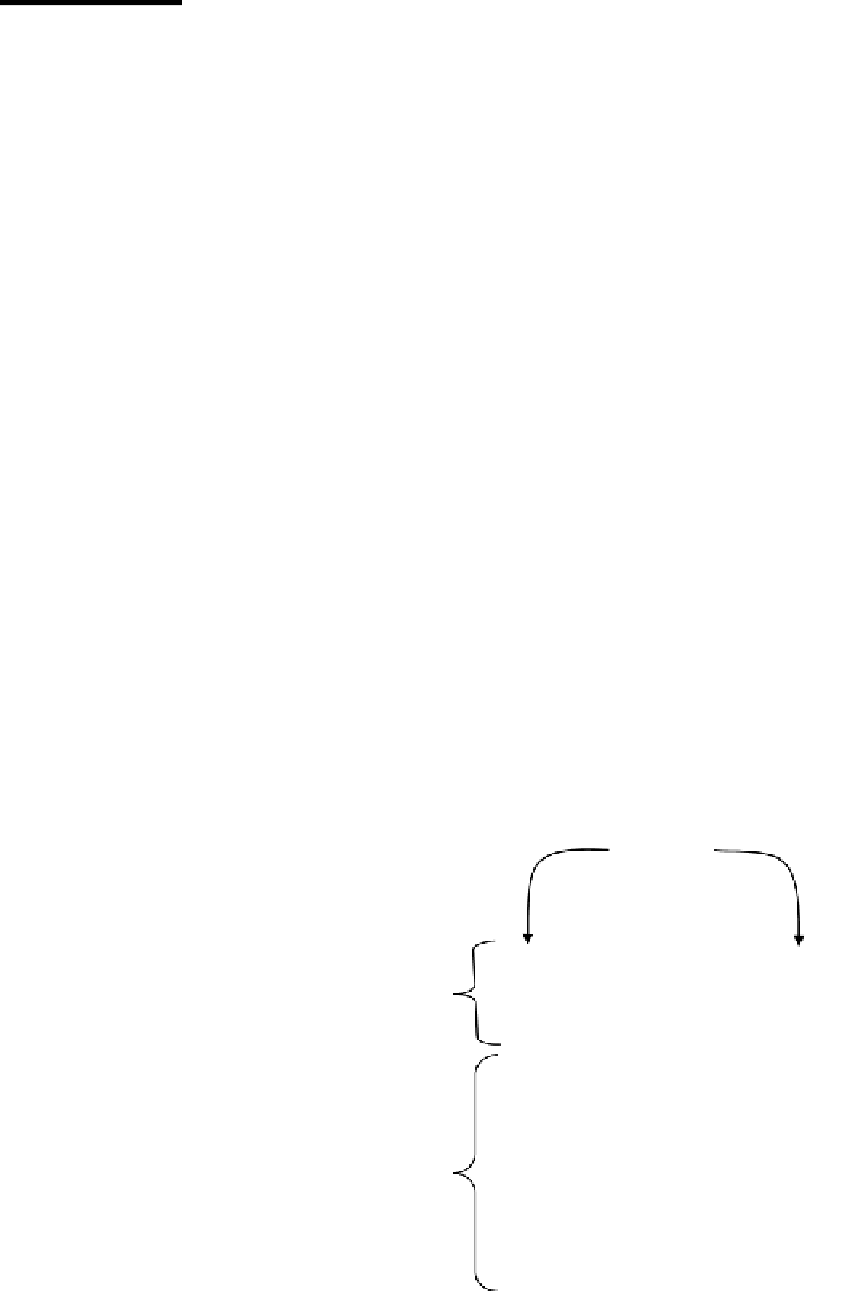
into the five following classes: IgG, IgM, IgA, IgD, and IgE. They have a Y-shaped structure
and are composed of two identical light chains and two identical heavy chains, which are
held together by disulfide bonds (2), as illustrated in Figure 20.1. Each heavy chain and each
light chain has a variable (V) domain and a constant (C) domain. The V domain is responsi-
ble for binding to the antigen, and the C domain mediates the antibody's function (2).
20.1.1
Immunoglobulin E
Human IgE is a monomeric immunoglobulin of 190,000 Da (3). Initially termed IgND after
its discovery by Johansson and Benich (4), it was soon confirmed to be identical to the
E
discovered by Ishizaka and Ishizaka (5), and determined to be central in the immediate
hypersensitivity reaction pathway (6). Of similar structure to the other immunoglobulins
(IgG, IgM, IgD, and IgA), it is composed of two light chains and two heavy chains, as
shown in Figure 20.2, and constitutes about 0.0005% of total serum immunoglobulins in
adults (3).
20.1.2
The Hypersensitivity Reaction
The term “allergy” was originally introduced by von Pirquet in 1906, meaning “changed
reactivity” of the host after the second or subsequent contact with an allergenic agent (7).
The first evidence of a transferable/soluble factor as the mediator of an allergic reaction
Ag
binding
site
Variable
region
S S
S S
Constant
region
Light
chains
Heavy
chains
FIGURE 20.1
General structure of an immunoglobulin (Ig).