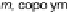Biomedical Engineering Reference
In-Depth Information
Viewed in this way, it can be thought that on going from self-assembled monomers to self-
assembled polymers, there would necessarily be an increase in the effective (
)
1/2
since
.
Amphiphilic phenolic and tyrosine-based monomers, possessing aliphatic decyl chains,
are capable of self-assembling into aggregates, beginning at their critical micelle concentra-
tions (cmc). We studied these monomers both electrochemically and using AFM. The DEDT
monomer (Figure 1.22), at concentrations below its cmc, was clearly shown to form films in
the EQCM device via electropolymerization, but it also adsorbed at significant levels onto
Pt electrodes in the absence of an applied potential (71). This did not occur with the tyro-
sine monomer, even at 100-fold higher concentrations. Using the EQCM, we further char-
acterized the concentration dependence of the DELT and DEDT isomer derivatives of
tyrosine (70). As monomer concentration was increased the films were found to grow
thicker until the cmc was reached, whereupon a saturation type of effect was observed.
Also, in the absence of monomer in solution the films formed above the cmc were found to
be unstable, undergoing a slow time-dependent desorption of adsorbed monomers from
the film. We also have studied copolymers formed from combining the monomers DELT
and
L
-tyrosineamide (79). As the Figure 1.28 schematic of the coupled self-assembly equi-
libria illustrates, aggregation occurs above the cmc of DELT, but it may not involve the
other monomer
L
-tyrosineamide. Above the cmc and before polymerization with HRP, the
DELT aggregate structures we observed in the comonomer mixtures possessed a smooth
amorphous morphology of varying shape and dimension, following their self-assembly
and adsorption to a gold surface. After HRP copolymerization, these complexes formed
structured interacting spherical aggregates upon the gold surface, as Figure 1.29 indicates,
that had diameters tightly centered around 1.4
certainly would increase on going from a monomer to a polymer and so might
m. Moreover, these structures now pos-
sessed obvious nanoscale surface structure. The self-assembled monomers and copoly-
merized structures also completely covered gold surfaces, even below their cmc, as
measured with x-ray photoelectron spectroscopy (88).
What is novel and important about the use of this QCM biosensor device for kinetic
measurements of a polymerization process is that these measurements can be performed
upon optically opaque solutions, where more widely used optical sensing techniques may
not be possible. Optical opacity was certainly the case for the self-assembling DELT sys-
tem we studied, since these measurements were performed well above the known cmc of
this monomer (87). Detection of the kinetics of the enzymatic polymerization process
relied upon the QCM device detecting an alteration in the underlying physical properties
C
10
H
21
C
10
H
21
C
10
H
21
NH
2
HRP
(H
2
O
2
)
O
NH
2
O
O
NH
2
=
C
C
C
O
=
C
C
C
O
C
C
C
=
O
C
C
C
=
O
=
=
C
C
C
O
C
C
C
O
m
H
HH
NH
2
H
HH
NH
2
H
HH
NH
2
H
HH
NH
2
m
H
HH
NH
2
n
H
HH
NH
2
+
m
cmc (pH)
+
n
n
, aggr
OH
OH
OH
OH
OH
OH
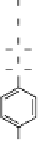
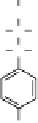
Delt
I
II
FIGURE 1.28
Schematic view of equilibria involved in the comonomer mixture of DELT and
L
-tyrosineamide including cmc-
dependent aggregation to form structure I and subsequent HRP copolymerization to form structure II. Structure II
is a projected copolymer state where the final copolymer linkages (
o
vs.
m
ring-ring linkages) have not been spec-
ified, since a mixture of the two is likely to occur. Reprinted with permission from Marx, K.A., Lee, J.S., Sung, C.
(2004). Enzymatic Copolymerization Alters the Structure of Unpolymerized Mixtures of the Biomimetic Monomers:
The Amphiphilic Decyl Ester of
L
-Tyrosine and
L
-Tyrosineamide—An AFM Investigation of Nano- to Micrometer
Scale Structure Differences.
Biomacromolecules
5:1869-1876. Copyright (2004) American Chemical Society.