Biomedical Engineering Reference
In-Depth Information
5
[
I
]=0.36 mM
4
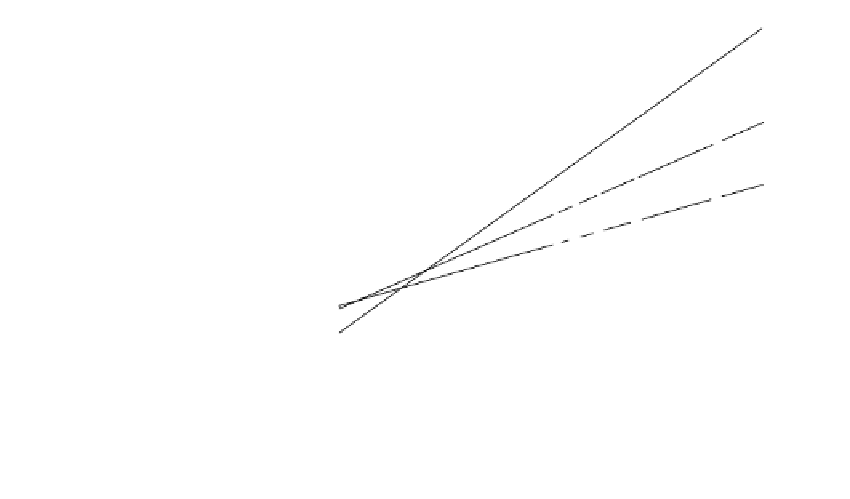
FIGURE 1.16
Lineweaver-Burk plot for paraoxon
(
I
)-mediated inhibition of alkaline phos-
phatase-catalyzed CSPD (
S
) reaction
rates (
v
). The reactions for different (
S
)
concentrations were studied at the three
different paraoxon (
I
) concentrations
indicated. Reprinted with permission
from Pande, R., Kamtekar, S., Ayyagari,
M.S., Kamath, M., Marx, K.A., Kumar,
J., Tripathy, S.K., Kaplan, D.L. (1996).
A Biotinylated Undecylthiophene
Copolymer Bioconjugate for Surface
Immobilization: Creating an Alkaline
Phosphatase Chemileuminescence-
Based Biosensor.
Bioconjugate Chem.
7:159-164. Copyright (1996) American
Chemical Society.
3
[
I
]=0.18 mM
2
[
I
]=0
1
0
0123
4
5
6
−
3
−
2
−
1
1/
S
(mM
−
1
)
−
1
−
2
−
3
indicating that this immobilized AP system exhibited the same type of mixed competitive
and noncompetitive inhibition with paraoxon as we demonstrated previously for AP in
solution studies (43). Furthermore, the immobilized AP system exhibited a robust charac-
ter, retaining 75% of the initial AP activity for at least 30 days when stored in buffer at 4°C
(44). As shown in Figure 1.17, this simple biosensor is sensitive to paraoxon inhibition,
especially at concentrations lower than 2 ppm, as the inset shows. We were able to achieve
lower detection limits of 500 ppb for paraoxon and 700 ppb for methyl parathion.
1.2.1.2.2 Detecting Zn
2
, Be
2
, and Bi
3
Ions Competitively
In solution studies, we further demonstrated that the AP-catalyzed CSPD chemileumi-
nescence biosensor system was capable of detecting certain metal ions (47,48). The AP
enzyme is a dimeric metalloenzyme containing four Zn
2
cations, two coordinated
within each of the AP's active sites. Therefore, we could determine Zn
2
by reactivation
of the alkaline phosphatase activity within the biosensor following prior Zn
2
removal
(48). The cations Zn
2
, Be
2
, and Bi
3
are all known to be inhibitors of native AP activity
(49-51). Therefore, their concentrations could be determined by their inhibition of the
native alkaline phosphatase within the biosensor. As an example, in Figure 1.18 we show
the inhibitory effects measured for the three cations on the AP system. Using the Zn
2
reactivation approach, sub-ppb Zn
2
sensitivities were achieved. Using the inhibition of
native AP, the detection limits achieved were 170 ppb for Zn
2
, 1 ppb for Be
2
, and 1.8
ppm for Bi
3
(47,48). Also, we showed that selective determination of Zn
2
in the pres-
ence of Be
2
could be achieved by masking the interfering ions with acetylacetone and
sodium fluoride. Although we did not pursue an immobilization strategy, we have
already demonstrated that successful copolymer-based immobilization of AP can be
achieved to form a chemileuminescence-based biosensor. This biosensor would likely be
sensitive for detecting these metal ions.
1.2.2
Electrochemical-Based Biosensors
There are distinct advantages that electrochemical methods provide over other signal trans-
duction methodologies for use in biosensors. One advantage is electrochemical control over